Lab Investigation 7 - Why is sodium acetate used in commercial hot packs and ammonium nitrate in commercial cold packs?
The Commercial Use of Thermochemistry
Guiding Question
Why is sodium acetate used in commercial hot packs and ammonium nitrate in commercial cold packs?Introduction
When a salt dissolves in water, two opposite heat transfer processes are involved. Energy is required to separate the associated ions in the solid salt, and energy is released as heat when the water molecules surround (solvate) the ions. Whether heat is liberated or absorbed overall depends upon the sum of the energies associated with these two processes. The reaction of dissolving a salt in water (dissolution reaction) is represented below. MA represents the salt, where M is any metal ion (+) and A is the non-metal ion (–).( 1 )
MA(s) → M+(aq) + A−(aq) (in H2O liquid)
The Problem
You and your teammates are to investigate different salts for potential use in cold or hot packs. Your job is to identify salts for use as reactants and provide guidelines regarding the mass of solid salt to be used in cold and hot packs. You will generate an argument using your quantitative data as well as other factors such as cost to justify the use of one salt for use in a cold pack and one salt for use in a hot pack.Materials available for use
- Electronic Balances
- Sodium acetate (NaC2H3O2)
- Ammonium nitrate (NH4NO3)
- Calcium chloride (CaCl2)
- Potassium nitrate (KNO3)
- Styrofoam cups
- Stir plates/magnets
- Vernier LabPro Data System
- T-85 Calculators
- Temperature probes
Safety Precautions
Caution:
Dispose of all salts properly.
Dispose of all salts properly.
Caution:
Wear goggles at all times.
Wear goggles at all times.
Getting Started
Each lab table has sodium acetate, used in hot packs, and ammonium nitrate, used in cold packs. Work in pairs to determine the heat of solution for each salt. For comparison purposes, test each salt twice. The procedure for quantitative heat investigations is below. You have two data tables for recording data and showing calculations.Class Poster Session
Once your group has completed the experiment, add your data to the class whiteboard.Report
This week you will NOT write a full lab report, just an argument for why each salt is used commercially. Your data is just one piece of information to consider, other factors to consider are solubility, toxicity, safety and cost (a quick web search will provide this information). In addition, you will each turn in your data tables with one set of calculations. Download and print the worksheet. You will use this worksheet to record your answers to these questions.Quantitative Heat Data for Salts
-
-
*qsoln = m × Cs × ΔTwhere m = mass in grams of water + salt
-
Cs= 4.18 J/g · °C, the same value as that of water.
-
Note: For all practical purposes, at constant pressure, q = ΔH.
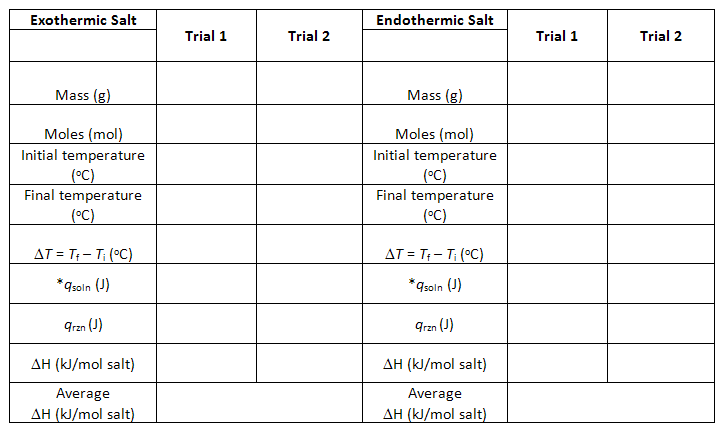
qsoln, qrxn, and ΔH
calculation for one Trial.
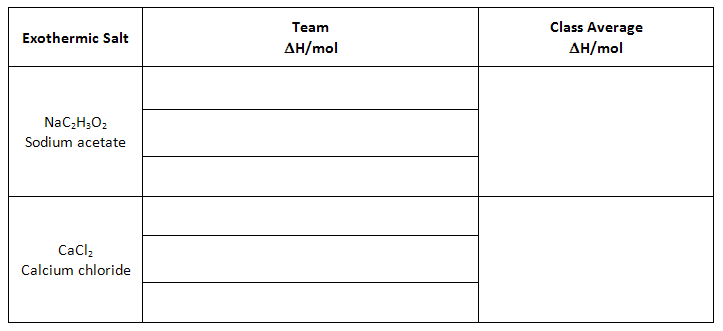
Class Data - Exothermic Salts
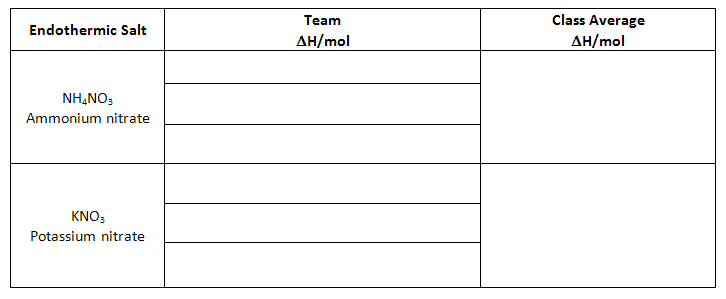
Class Data - Endothermic Salts
Recommended procedure for quantitative heat investigations
-
•A calorimeter can be constructed from two concentric Styrofoam cups. This is done to minimize heat exchange with the surroundings.
-
•Use two concentric Styrofoam cups with a temperature probe and a magnetic stirrer inserted into the cup through a cardboard or plastic lid. (See Figure 1)
-
•Make sure that the probe does not touch the bottom or the sides of the calorimeter.
-
•It is important to use the same amount of distilled water in each trial. We suggest 100. mL.
-
•To measure the heat absorbed or liberated, simply add a desired (4 to 8 grams recommended) amount of a salt to the water, stir, and then find the initial and final temperatures of the water.
-
•Be sure to conduct repeated trials to reduce measurement error. We would suggest you use a different mass for the second trial.
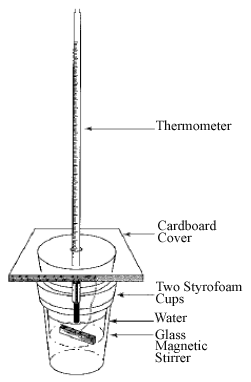
Figure 1: The Calorimeter