Lab 4 - Qualitative Analysis
Purpose
To develop a separation scheme and confirmatory tests for Fe3+, Ba2+, and Ag+ cations and to use the scheme and tests to identify the ions in a sample of unknown composition.Goals
-
1To explore solubilities and reactivities of different metal ions.
-
2To identify ions present in unknown solutions using separation methods.
-
3To gain experience with logically developing a qualitative analysis scheme.
Introduction
Qualitative analysis is the process by which components of mixtures are separated and identified. Unlike quantitative analysis, where the amount of a particular material is measured, a qualitative analysis scheme simply confirms the presence or absence of certain materials. A common analysis is the identification of aqueous ions. In such an analysis, there are two distinct phases. First, a scheme must be developed to separate the ions from each other. Then, a different test is performed to uniquely confirm the identity of each separated ion. In this lab, we develop a qualitative analysis scheme to separate and identify the components of a chemical mixture. The mixture will be composed of the iron (III) ion (Fe3+), the barium ion (Ba2+), and the silver ion (Ag+). In addition to analyzing the unknown ion mixture for its component ions, the development of a qualitative analysis scheme highlights some of the important chemical behavior of these metal salts in aqueous solution. The principles of chemical equilibrium are emphasized, as illustrated by precipitation reactions, acid-base reactions, complex-ion formation, and oxidation-reduction reactions Ions are often separated in solution by their different solubilities. A metal ion in a mixture may precipitate (form a solid) in the presence of a specific anion, while the remaining metal ions remain dissolved (in aqueous form). The two ions may be separated by collecting the solid at the bottom of a test tube in a centrifuge, a device that creates a centrifugal force by rotation. After the precipitate is compacted, the supernatant (the liquid solution above the solid) is decanted (carefully poured off) into a separate container. The soluble ion is in the liquid supernatant while the insoluble ion is in the solid precipitate. Thus they are physically separated from each other when the liquid is poured off, leaving the solid behind. The solid precipitate is typically washed with water to help remove any traces of the soluble ions that remain. This prevents "false positive" test results later on. Once the ions in a mixture have been separated, their identity can be further verified by a confirmatory test. In a confirmatory test, each ion has a unique response to an added chemical, such as a solution color change or the formation of a precipitate. This unique response confirms the presence of that particular ion.Basis of the Qualitative Analytical Separations in this Scheme
The separations used in this qualitative analytical scheme are based on the facts contained in an abbreviated set of solubility rules (see D. L. Reger, S. R. Goode and E. E . Mercer, "Chemistry Principles and Practice," Second Edition, Saunders College Publishing (Phila. PA) 1997, p 137).
Brief Solubility Rules
-
1All nitrates are soluble.
-
2All chlorides are soluble except AgCl, Hg2Cl2, and PbCl2.
-
3All sulfates are soluble except SrSO4, BaSO4, CaSO4, and PbSO4.
-
4All carbonates are insoluble except those of the group IA elements and NH41+.
-
5All hydroxides are insoluble except those of the group IA elements and NH41+, Sr2+, and Ba2+. Ca(OH)2 is slightly insoluble.
-
•Insoluble compounds here are defined as those which precipitate upon mixing equal volumes of solutions 0.1 M in the corresponding ions.
- Group 1: HCl is added to the soluble nitrate salts of the metal ions (rule 1) to precipitate AgCl (rule 2).
- Group 2: Addition of NH3 (also known as NH4OH) makes the solution basic and precipitates the hydroxide of Fe3+ (rule 5). A portion of the added NH3 reacts with the HCl from the first separation to form NH41+ (ammonium cation), forming a buffer solution with the unreacted NH3.
- Group 3: BaSO4 is precipitated by adding sulfuric acid (rule 3).
General Techniques
The qualitative analysis scheme that follows is carried out on what is called a semimicro (small) scale of operations. This requires minimal quantities of reagents and sample, and the time necessary to carry out the operations is much less than that required using a macroscale (large) set of operations. The indications of reaction, appearance of a precipitate, development of color, etc., are all readily discernible on this semimicro scale. The usual reactor vessel on this scale is the 10 × 75 mm test tube. The quantities of solution handled are of the order of one to two milliliters, and the volumes of reagents to be added are on the order of one to several drops(~0.05-0.5 mL). Convenient reagent containers to use at the laboratory bench consequently are 12 to 15 mL screw cap bottles equipped with rubber-capped medicine droppers.
Separation of Precipitates from Solutions
The principal separation method used is precipitation. This separation is performed using a centrifuge, which spins the sample in a small test tube at high speeds, causing the precipitate to settle rapidly to the bottom of the tube. In using the centrifuge,-
1Always counterbalance the sample tube with a test tube containing water filled to the same level as the sample.
-
2Always lower the centrifuge cover before turning it on (If a tube breaks, those nearby won't be hit by flying glass.).
-
3Allow the centrifuge to coast to a stop. Do not attempt to slow it with your fingers
Washing a Precipitate
Since decantation does not completely remove the solution, it is recommended that the precipitate be washed following the separation. Washing is done by placing about 1 mL of water (or a recommended washing solution) into the tube containing the precipitate. Stir with a vertical motion with a stirring rod to suspend the solid in the washing solution. Centrifuge and decant the liquid.Addition of Reagents
The reagents are measured in drops delivered from a dropper bottle. When adding reagents, the tip of the dropper must never touch the test solution or the walls of the test tube. A contaminated reagent solution can cause erroneous results.Test for Completeness of Precipitation
After adding the specified amount of a precipitating reagent, it is suggested that a test for completeness of precipitation be carried out as follows. Centrifuge and, without decanting the solution, add a drop of reagent so it runs down the wall of the test tube. If precipitation is complete, no new precipitate will form when the reagent dissolves in the solution.Re-Precipitation
In some cases, particularly when the precipitate (ppt.) is gelatinous in nature, it may have to be dissolved and re-precipitated to separate salts that may have been carried down with the gelatinous precipitate. The resulting solution, after centrifuging and decantation, is added to the solution resulting from the first precipitation or discarded.Recording Observations
All observations and conclusions should be recorded on your lab data sheet.Experiment Summary
In Part A, you will run confirmatory tests on the individual ions (Fe3+, Ba2+, Ag+) to determine their unique behavior in the presence of hydrochloric acid, sodium thiocyanate, and sulfuric acid. In Part B, you will perform a qualitative analysis scheme designed to physically separate these ions and confirm the results. In Part C, you will use the same qualitative analysis scheme to separate and identify the cations in an unknown mixture.Equipment
-
6small test tubes
-
1test tube rack
-
1test tube brush
-
3glass stirring rods
-
1250 mL beaker for waste collection
-
1plastic 4 × 6 well plate
-
1centrifuge
-
1deionized water squirt bottle
Reagents
In dropper bottles:- 0.10 M Fe(NO3)3
- 0.10 M Ba(NO3)2
- 0.10 M AgNO3
- unknown solutions
- deionized water
- 6 M HCl
- 6 M H2SO4
- 6 M HNO3
- 7.5 M NH3
- 0.05 M NaSCN
Safety
HCl, H2SO4, HNO3 and NH3 are corrosive. They can attack the skin and cause permanent damage to the eyes. If one of these solutions splashes into your eyes, use the eyewash immediately. Hold your eyes open and flush with water. If contact with skin or clothing occurs, flush the affected area with water. Have your lab partner notify your instructor about the spill. The solutions also have irritating vapors. They should be used in a fume hood; avoid inhaling the vapors. HCl, H2SO4, and HNO3 are strong acids, and NH3 (ammonia) is a base. Silver solution will form dark spots on skin if spilled. The spots will not appear for about 24 hours, as the ions are slowly reduced to the metal. They are not hazardous, and will fade in a few days. Students will have access to gloves due to the use of concentrated acid and base solutions during the lab period.Waste Disposal
All solutions used or produced in this experiment must go into the waste container, as they all potentially contain heavy metal cations. Label a 250 mL beaker as a waste container for use at your bench. Empty the beaker into the lab waste container in the hood when you have finished your work, then rinse it with a few mL of water. These rinsings should also go into the lab waste container. Afterwards, the test tubes can be washed with soap and water in the sink. The precipitates should also go into the waste container. Nothing should go into the sink.Prior to Class
Please read the following section of the Introductory Material. Please complete the WebAssign prelab assignment. Check your WebAssign account for due dates. Students who do not complete the WebAssign prelab assignment are required to bring and hand in the prelab worksheet.Lab Procedure
Please print the worksheet for this lab. You will need this sheet to record your data.Part A: Confirmatory Tests for Individual Ions
1
In a 4 × 6 plastic well plate, prepare a three by three grid of cation solutions and confirmatory reagents. In the first column, place three drops of iron ion solution in all three wells. In the second column, place three drops of barium ion solution in all three wells. The third column will be composed of three drops of silver ion in the three wells.
2
In the first row, add two drops of 6 M HCl solution to each cation solution and record your observations (precipitate, color change) in Data Table A.
3
In the second row, add one drop of 0.05 M NaSCN to each cation solution and record your observations.
4
In the third row, add two drops of 6 M H2SO4 to each cation solution and record your observations.
5
You may retain the well plate during the remainder of the experiment to compare known results with your unknown solutions.
Data Table A: Confirmatory Tests for Individual Ions
Question 1: Which ion(s) precipitate when HCl is added?
Question 2: Which ion(s) precipitate when H2SO4 is added?
Part B: Qualitative Scheme for a Mixture of All Three Ions
Note: As you work, document observations regarding the color of precipitates and solutions in the Figure 1 Qualitative Separation Scheme on your lab worksheet. These observations will aid you in identifying the components of the unknown mixture in Part C.
1
Add three drops of each cation solution (Fe3+, Ba2+, and Ag+) to a single test tube.
2
Add a few drops of 6 M HCl. A precipitate will appear. Continue adding HCl until no more solid appears to be forming. Centrifuge the sample.
3
Add another drop of HCl. If no precipitate forms, go to step 4. If more solid appears, add HCl until no more forms. Centrifuge again, and add another drop of HCl. Continue this until no more solid forms, then go to step 4.
Question 3a: Based upon your observations from Part A, which ion(s) precipitate when HCl is added?
Question 3b: Which ions(s) remain in the supernatant?
4
Decant the supernatant liquid into a clean test tube. The solid in the original test tube can be labeled (ppt. #1) and set aside for later testing.
5
To the supernatant from step 4, add 10 drops of 7.5 M NH3. A precipitate will form. Continue adding NH3 until no more solid forms. Centrifuge the sample. Be sure to test with one additional drop of 7.5 M NH3 to be sure that no additional solid forms.
Question 4a: Based upon the solubility rules in the introduction, which ion(s) remaining in the supernatant from step 4 will precipitate in basic solution?
Question 4b: Which ion(s) remain in the supernatant from step 5?
6
Decant the supernatant liquid into a clean test tube. The solid in the test tube can be labeled (ppt. #2) and set aside for later testing.
7
To the supernatant from step 6, add two drops of 6 M H2SO4. A precipitate will form. Continue adding H2SO4 until no more solid forms. Centrifuge the sample. Be sure to test with one additional drop of 6 M H2SO4 to be sure that no additional solid forms.
Question 5a: Based upon your observations in Part A and logic, which ion(s) remaining in the supernatant from step 6 will precipitate when H2SO4 is added?
Question 5b: Which ion(s) remain in the supernatant from step 7?
All three ions (Fe3+, Ba2+, and Ag+) have now been physically separated. The location of each ion can now be confirmed using the reagents from Part A.
8
Take the test tube containing ppt. #1 from step 4 and wash it with cold water by adding ~0.5 mL of cold water and breaking up the solid with a glass stir rod. Centrifuge the sample and decant the supernatant liquid into a waste container. Retain the solid for the next step.
9
Add three drops of 7.5 M NH3 and another 0.5 mL of cold water. Again, break up the solid with a glass stir rod. Centrifuge the sample and decant the supernatant liquid into a clean, dry test tube. This time, the precipitate can be discarded into the waste container.
10
To the supernatant from step 9, add 3 drops of 6 M HNO3. A precipitate should form. Check Data Table A for the ion that precipitates in the presence of Cl–, which is still present in the mixture from step 9.
11
Take the test tube containing ppt. #2 from step 6 and add three to five drops of 6 M HCl until the solid dissolves. Then add one drop of 0.05 M NaSCN. Check Data Table A for the ion that has a unique appearance in the presence of NaSCN.
Question 6: Use the data you have acquired in Parts A and B to complete the flow chart below. It will serve as a reference for identifying the cations in an unknown solution in Part C.
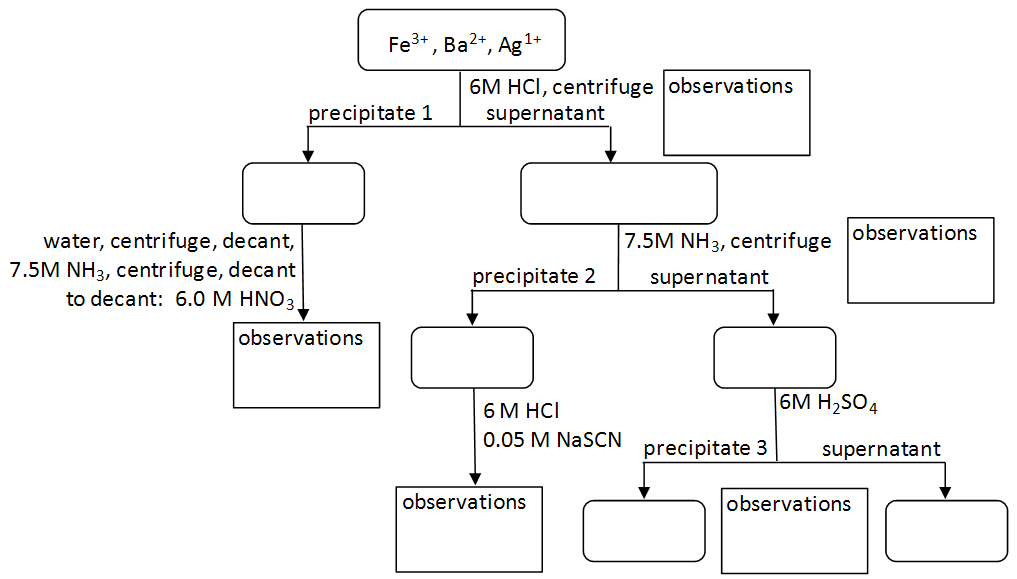
Figure 1: Qualitative Separation Scheme and Confirmation of Fe3+, Ba2+, and Ag+
Question 7: Describe the location of each cation in the separation scheme and the confirmatory test used to confirm the presence of that ion.
12
Discard the contents of the test tubes in the waste container and rinse the test tubes thoroughly with deionized water.
Part C: Identifying the Cations in an Unknown Mixture
Note: As you work, document observations regarding the color of precipitates and solutions in the Figure 2 Qualitative Separation Scheme on your lab worksheet. Compare the observations from Parts B and C to assist in identifying the components of the unknown mixture in Part C.
1
Add nine drops of an unknown solution into a clean test tube. Enter your unknown number at the top of the Figure 2 Qualitative Separation Scheme on the lab data sheet.
2
Repeat steps 2-12 from Part B, documenting your observations as you
work in Figure 2 found in the lab worksheet.
3
Before leaving, go to a computer in the laboratory and enter your results in the InLab assignment. If all results are scored correct, log out. If not all results are correct, try to find the error or consult with your teaching assistant. When all results are correct, note them and log out of WebAssign. The InLab assignment must be completed by the end of the lab period. If additional time is required, please consult with your teaching assistant.
Question 8: Complete the flow chart below for your unknown solution, identifying which (if any) ions are present at each branch and endpoint in the chart.
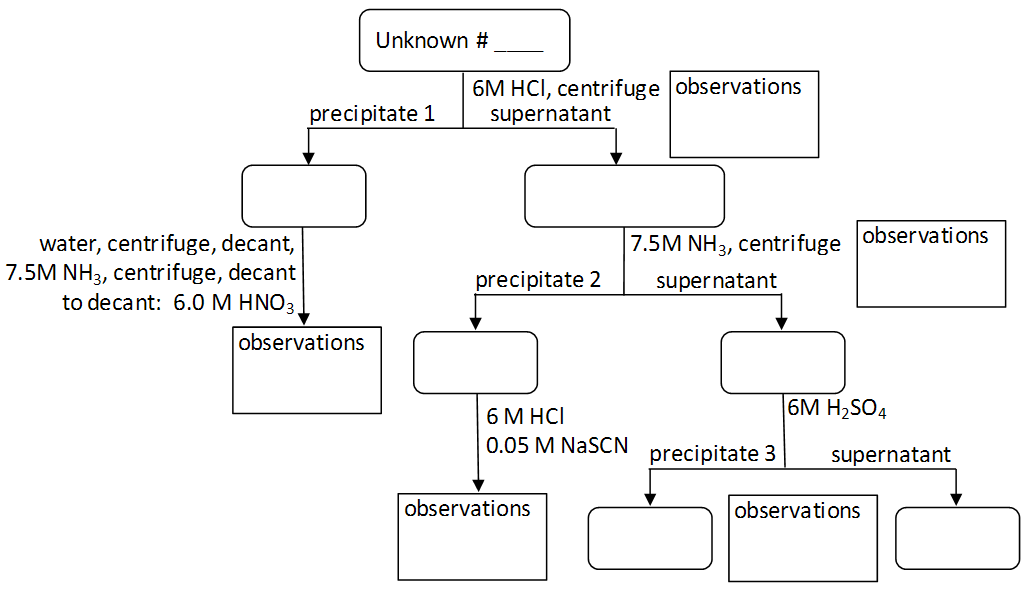
Figure 2: Qualitative Separation Scheme Template for Unknown Mixture
Question 9: Write the net precipitation reaction that occurs when HCl is added to an aqueous solution containing Fe3+, Ba2+, and Ag+ ions.
Question 10: Write the net precipitation reaction that occurs when a solution containing Fe3+ and Ba2+ is made basic (contains OH–) from the addition of NH3.
Question 11: Write the net precipitation reaction that occurs when H2SO4 is added to a solution containing Ba2+.

