Module 4 – Density
Introduction
What do ballpoint pens, armor-piercing bullets, fingerprints, DNA, and the boat below have in common? All of these topics are related to density. Osmium is the most dense of all elements, and it is often combined with other metals to form extremely hard alloys used to make ballpoint pens and armor-piercing bullets. Osmium tetroxide is used by forensic scientists to detect DNA and fingerprints. Mass density is a physical property that determines whether boats will sink or float.
Figure 1
Density
Why doesn't it make sense to say that lead is heavier than water? The answer, of course, is that it depends on how much water and lead you have. For example, the weight of 1 cubic foot of lead is 708 lb, but the weight of 12 cubic feet of fresh water is about 750 lb. When someone says that lead is heavier than water, they are really trying to say that lead is more dense than water. They mean that when they have the same volume of lead and water, then lead will be heavier. Mass density is the physical quantity that relates the mass (or the weight) of a substance to its volume. Mass density is defined as follows. Weight density is defined as follows.
Density and Crystal Structure
The Sl units of mass density are kg/m3, but there are several other common units. One of the most commonly used units of mass density is gram per cubic centimeter, or g/cc. This is because pure water has a mass density of 1 g/cc. It turns out that 1 mL of liquid is equal to 1 cc of volume. So it is also possible to express the mass density of water as 1 g/mL. This makes water a useful tool since it is possible to use graduated cylinders to measure volumes. Take a look at the following elements and their densities. Notice that osmium has the smallest atomic number, which means that it has the fewest protons in its nucleus, but notice that osmium's mass density is the largest. So, apparently, the mass of an element does not determine its density. If it did, then lead should have the greatest mass density of these three elements.
Figure 2
Measuring Mass Density
Suppose you need to measure the mass density of an irregular object. Say that you are Archimedes, and the King of Syracuse has asked you to determine whether a craftsman has made a crown of silver rather than gold. What would you do? If you were Archimedes, you'd invent a procedure! The first step is to measure the volume of the crown by immersing it into water. The amount of water displaced is equal to the volume of the crown. Then all you'd need to do is weigh the crown and apply the definition for mass density. Let's try it!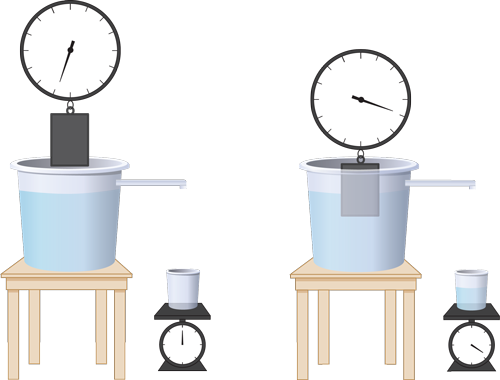
Figure 3
| Mass Density | = |
| |||||
| = |
| Equivalently, you could say =
| |||||
| = | 10.5 g/mL = 10.5 g/cc | (which is equivalent to 10.5 kg/L) | |||||
Will it float or sink? Ask Archimedes!
From your reading, you should recall Archimedes' Principle, as described below (Ostdiek and Bord 2018, 148).
Figure 4
The condition for floating is that the buoyant force is greater than the weight of the object. In equation form, this is:
- To Float: weight of object < weight of displaced fluid.
-
mass density of object × object volume < mass density of fluid × object volume mass density of object < mass density of fluid
PROCEDURE
This experiment consists of three parts.1
Open the experiment instructions and worksheet.
-
•Density Experiment Instructions (HTML or PDF)
-
•Density Experiment Worksheet
2
After you have thoroughly read the instructions and worksheet, open the experiment simulation in which you will conduct the experiment and collect your data.
3
Record your data in the worksheet. (You will need it for the lab report assignment in WebAssign.)
