Lab 3 - Extraction
Objective
In this experiment, you will separate the components of a commercial headache powder via an extractive process. This separation will be accomplished by taking advantage of the fact that each component contains different functional groups which will react differently when treated with a specific reagent.Introduction
Extraction is a widely used method for the separation of a substance from a mixture. It involves the removal of a component of a mixture by contact with a second phase. Solid-liquid and liquid-liquid extractions are commonly performed by batch and continuous processes. The removal of caffeine from coffee beans with dichloromethane is an example of a solid liquid extraction. Crystal violet may be removed from a water solution by liquid-liquid extraction with n-amyl alcohol (1-pentanol). Other common applications of liquid-liquid extractions involve:-
1Isolation of organic reaction products
-
2Removal of acid, base, and salt impurities
-
3Removal of organic acids and bases from other organic compounds
( 1 )
Kp =
| concentration of A in S (g/mL) |
| concentration of A in S' (g/mL) |

Figure 1
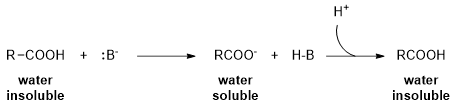
Figure 2
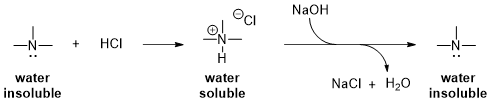
Figure 3
Procedure
1
Before you start, watch the liquid-liquid extraction video.
2
In a 50 mL Erlenmeyer flask, dissolve 1 g of headache powder (the contents of one packet) in 20 mL of diethyl ether. The headache powder contains some binders which are insoluble in ether. All of the powder may not dissolve, but this is not a problem.
3
Make sure the stopcock on the separatory funnel is closed.
4
Pour the solution into the separatory funnel.
5
Use a fresh 5 mL of diethyl ether to transfer the remaining contents of the Erlenmeyer flask to the separatory funnel.
6
Measure approximately 20 mL of a 5% sodium bicarbonate (NaHCO3) solution.
7
Transfer the bicarbonate solution to the separatory funnel.
8
Stopper the funnel, invert the funnel, and release the pressure by opening the stopcock.
9
Continue extraction by shaking, inverting, and venting until no audible or visible gas emerges (i.e. nothing comes out of the stopcock when releasing pressure).
10
Place the separatory funnel in an iron ring on a ring stand and remove the stopper immediately.
11
Remove the aqueous layer (which is it, top or bottom?) into a labeled beaker. Be careful and slow to dropwise flow as the level of the layers lowers in the funnel. Be sure to leave a drop of the bottom layer in the separatory funnel. This solution contains the conjugate base of acetylsalicylic acid.
12
Obtain 1-2 mL of 6 M HCl in a small test tube and a Pasteur pipet.
13
Cool the Sodium Bicarbonate Layer that you just extracted in an ice bath.
14
While still in an ice bath, carefully acidify this solution by slowly adding the 6 M HCl dropwise until no additional acetylsaliylic acid solid is produced. Add the acid slowly since CO2 will be produced and effervescence will occur.
15
Once the solution is acidic, collect the precipitate by vacuum filtration. You can watch a short video that describes the vacuum filtration set up.
-
aClamp the filter flask to a ring stand.
-
bConnect filtration assembly to vacuum.
-
cPut neoprene seal on the mouth of the flask.
-
dPut Buchner funnel in the seal.
-
ePut the filter paper in the funnel.
-
fWith vacuum running, squirt water on the entire surface of the filter paper to seat paper.
-
gSlowly, pour the solution into the center of the funnel.
-
hIf necessary, transfer the remaining solid from beaker with a spatula.
-
iFor best yield, refilter the filtrate (i.e. the liquid in the filter flask) if it is cloudy.
16
Dry your filtered acetylsalicylic acid.
-
aSeparate paper from funnel with spatula.
-
bScrape solid off of paper into a small beaker.
-
cDry in oven for ten minutes.
17
Once you have isolated the acetylsalicylic acid, make sure to dispose of the ether layer in the waste container provided. Ask your TA if you have any questions.
Determining the Melting Point
1
You can watch a short video that describes how to determine a melting point using a DigiMelt apparatus.
2
Loading the melting point capillary:
-
aPlace the open end of the capillary tube gently into the substance several times to achieve a sample height of 2 to 3 mm inside the capillary tube.
-
bThe sample is pushed to the bottom of the capillary tube by tapping the bottom against a hard surface via a drop tube.
-
cPlace the capillary tube in the DigiMelt sealed end down.
-
dLoad samples from three groups before running the DigiMelt in order to minimize time spent running Melting Points.
3
Following the steps on the front of the instrument (read them carefully), set the starting temp 30°C below the theoretical melting point, the ramp rate at 5°C/min, and the end temp 10°C above the theoretical melting point.
4
To record the start of the melt temperature and the end of melt temperature press the number of the sample as you reach each temperature.
5
Instructions are on the DigiMelt for recalling these values.
In-Lab Questions
Please print the worksheet for this lab. You will need this sheet to record your data.Questions
1
Amount of Acetylsalicylic acid in powder (see box or bulk container) ___________________
2
Amount of Acetylsalicylic acid recovered ___________________
3
Percentage Recovery _______________
4
Melting Point of Acetylsalicylic Acid _______________ (observed)
Melting Point of Acetylsalicylic Acid _______________ (lit. reported)
5
A. Which of the compounds in the BC powder is the most acidic? The most basic?
B. Draw the compounds obtained for each step of the extraction in the boxes provided in the experiment in the following flowchart.
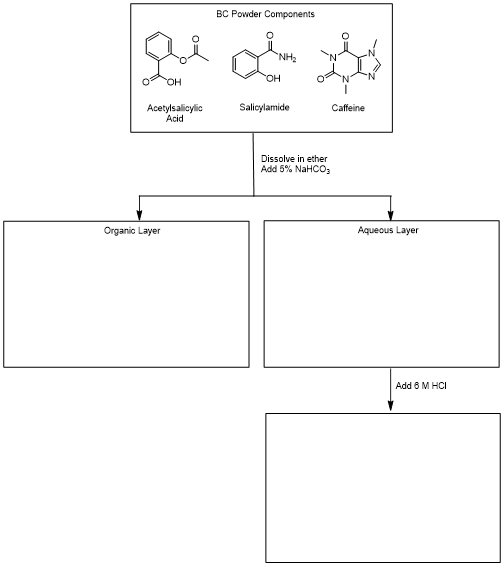
Figure 6

