Lab 9 - Titrations
Purpose
To determine the concentration of acetic acid in vinegar.Goals
-
•To perform an acid-base titration.
-
•To gain experience titrating carefully to a visible endpoint.
-
•To calculate the amount of analyte present from the result of a titration.
Introduction
Many laboratories analyze consumer products to determine accuracy in the labeling of the product. The very common and simple technique of titration is demonstrated in this experiment. A titration is an analytical procedure in which a reaction is run under carefully controlled conditions. The stoichiometric volume of one reactant of known concentration, the titrant, that is required to react with another reactant of unknown concentration, the analyte, is measured. The concentration of the analyte is determined from the concentration and volume of titrant and the stoichiometry of the reaction between them. The experimental setup is shown in Figure 1. A buret, which contains the titrant, is calibrated so the volume of solution that it delivers can be determined with high accuracy and precision. Titrant is added to the analyte until the stoichiometric volume of titrant has been added. This is called the equivalence point, at which the volume of titrant delivered by the buret is read. Usually, the volume readings are estimated to the nearest 0.01 mL. The delivery of the titrant is adjusted with the stopcock on the buret. With practice, one can dispense fractions of a drop of titrant and control the procedure well enough that replicated titrations agree within 0.10 mL. For this first lab, you will need your titrations to agree to within 0.50 mL.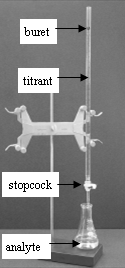
Figure 1: Titration Setup
(gacid = MWacid x molesacid). Finally, the mass percent of acetic acid in the vinegar can be determined from the mass of the acetic acid in the sample and mass of the vinegar solution that was titrated. In the titration of acetic acid with aqueous NaOH, phenolphthalein is used as the indicator. Phenolphthalein is nearly colorless in acidic solution, but turns pink at a pH of about 8. This indicates that the base has neutralized all the acid. As you titrate the vinegar, you will observe that the pink color is more persistent as you add more base. This is a signal to slow the addition of base, and control it carefully. The endpoint has been reached when a faint pink color persists for at least 30 seconds. It is easy to overshoot the endpoint. If this happens, you will have a dark purple-pink solution, and you will have to repeat the titration, so be careful. Note the volume you have used; stop short of this volume in subsequent titrations, and add the last milliliter or so dropwise. Your instructor will show you how to control the stopcock of the buret to facilitate this. Note that the volume measurements in titrations are usually reported to four significant figures, so the concentrations are usually reported to four significant figures as well. Watch this in your work; when you calculate molar masses, make sure you have four significant figures.
Equipment
- 1 10.0 mL graduated cylinder
- 1 30 mL beaker
- 1 100 mL beaker
- 1 25 mL buret
- 1 ring stand and buret clamp
- 2 125 mL Erlenmeyer flasks
- 1 deionized water squirt bottle
Reagents
- ~50 mL 0.5 M sodium hydroxide (NaOH)
- ~25 g commercial vinegar (HC2H3O2)
- ~1 mL phenolphthalein solution
- deionized water
Safety
NaOH is corrosive. It can attack the skin and cause permanent damage to the eyes. If NaOH solution splashes into your eyes, use the eyewash immediately. Hold your eyes open and flush with water. If contact with skin or clothing occurs, flush the affected area with water. Have your lab partner notify your instructor about the spill.Waste Disposal
All solutions can be flushed down the sink with plenty of water.Prior to Class
Please read the following sections of the Introductory Material: Analytical Balance, Volumetric Glassware, and Measurements. Please review the following videos: Cleaning, conditioning and filling a buret, Performing a titration, and Cleaning and storing a buret. Please complete WebAssign prelab assignment. Check your WebAssign Account for due dates. Students who do not complete the WebAssign prelab are required to bring and hand in the prelab worksheet.Lab Procedure
Please print the worksheet for this lab. You will need this sheet to record your data.-
1Obtain a clean, dry 10.0 mL graduated cylinder.
-
2Using a clean, dry 30 mL beaker, obtain about 25 mL of vinegar.
-
3Condition the graduated cylinder with vinegar solution before using it. This is done by adding a little vinegar solution to the graduated cylinder, swirl so that all of the sides are coated with vinegar and then discarding the remaining vinegar. Repeat this procedure 1–2 more times to ensure that the graduated cylinder is conditioned.
-
4Obtain about 50 mL of 0.5 M NaOH solution in a clean, dry 100 mL beaker. Record the exact concentration of NaOH in Data
Table A1. -
5Condition the 25.0 mL buret with NaOH solution as directed by your instructor, and according to the description in "Volumetric Glassware" in Lab Equipment.
-
6Fill the buret with NaOH and carefully clamp it to the ring stand.
-
7Record the initial reading on the buret, to the nearest 0.01 mL in Data Table A1. Reading a buret to this accuracy is tricky. Remember, the last significant figure is expected to be an estimate.
-
8Measure the mass of an empty 125 mL Erlenmeyer flask and record this value in Data Table A1. Using the 10.0 mL graduated cylinder, transfer 10.0 mL of vinegar into the flask. Weigh the flask and vinegar together and record the mass in Data Table A1.
-
9Add ~10 mL of deionized water (Do not use the graduated cylinder for this now that it is conditioned for vinegar!) and 3 drops of phenolphthalein solution to the flask containing the vinegar.
-
10Open the stopcock of the buret and add some titrant (NaOH) to the contents of the flask (HC2H3O2 + water + indicator). Swirl the flask gently to mix the solutions.
-
11Continue to add titrant to the flask with swirling. As the addition proceeds, you will see a faint pink color appear and quickly fade. When the color begins to disappear more slowly, slow the addition of titrant to a dropwise rate. Rinse the walls of the flask and the tip of the buret with deionized water from a wash bottle as you approach the endpoint. This ensures that all of the NaOH delivered from the buret ends up in the reaction mixture. The endpoint has been reached when the faint pink color lasts for at least 30 seconds. Record the final reading on the buret to the nearest 0.01 mL in Data Table A1.
-
12Repeat steps 6–11 with a second sample of vinegar. If the results of the two trials differ by more than 0.50 mL, titrate a third sample. You will need to clean one of your Erlenmeyer flasks to perform a third titration. Discard the trial with the darker pink color. For the third titration, try match the color of the remaining endpoint as well as you can.
-
13When finished, drain the remaining NaOH from your buret into your 100 mL beaker. Discard all solutions in the sink with plenty of water.
-
14Rinse all of your glassware with water, dry it and return it to the set-up area where you found it.
-
15Before leaving, go to a computer in the laboratory and enter your results in the In-Lab assignment. If all results are scored as correct, log out. If not all results are correct, try to find the error or consult with your lab instructor. When all results are correct, note them and log out of WebAssign. The In-Lab assignment must be completed by the end of the lab period. If additional time is required, please consult with your lab instructor.