Lab 5 - Molecular Geometry
Purpose
-
ATo explore some simple molecular structures
-
BTo explore the relationship between bond order and bond length
-
CTo explore resonance structures
Goals
-
•To compare Lewis structures to three-dimensional models
-
•To visualize the three-dimensional structures of some common molecules
-
•To obtain bond angle, bond length, and hybridization data for molecules
-
•To rationalize differences in predicted and measured values
-
•To learn how to use computer modeling software
Introduction
The chemical and physical properties of covalently bonded materials are related to the spatial arrangement of the atoms and other electrons not involved in the actual bond formation. There are many ways to depict the spatial arrangement in both two and three dimensions. A Lewis structure is a two-dimensional representation of the arrangement of the atoms, bonding electrons and non-bonding (lone pair) electrons in a covalent material. In a Lewis structure, the nucleus is represented by the atomic symbol with a line between the atoms in a bond depicting each pair of shared bonding electrons in the structure. Non-bonding electrons around the atoms are depicted as dots. The steps to building a Lewis structure representation of a molecule are shown below. The Lewis structure of the formate ion, CHO2–, will be used as an example.1
Calculate the electrons required (ER) = the minimum number of electrons
necessary to satisfy the octet rule for the non-hydrogen atoms and the duet rule for hydrogen. For CHO2–, this would be (2 electrons × 1 hydrogen atom) + (8 electrons × 3 non-hydrogen atoms) = 2 + 24 = 26 electrons required.
2
Calculate the number of available valence electrons (VE) = the total
number of electrons available for the molecule. For example, in CHO2–, this would be (1 C atom × 4 electrons) + (1 H atom × 1 electron) + (2 O atoms ×
6 electrons) + (1 electron as the ion has a charge of –1) = 4 + 1 + 12 + 1 = 18 valence electrons. NOTE: For ions, the charge must be included in this by adding the charge on an anion or subtracting the charge on a cation.
3
Calculate the Shared Pairs (SP) = the number of electrons to be shared in
bonds. The SP is 1/2(ER – VE); for CHO2–, this would be 1/2(26 – 18) = 4 shared pairs or four bonds.
4
Calculate the Lone Pairs (LP) = the number of electron pairs belonging to
only one atom. The LP is 1/2(VE – (2 × SP)); for CHO2–, this would be 1/2(18 – (2 × 4)) = 5 lone pairs. Notice that VE = 2 × (SP + LP).
5
Place the first atom in the molecular formula as the central atom,
surrounded by the other atoms in the compound.
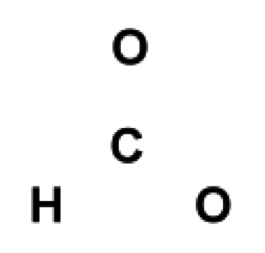
Figure 1
6
Draw bonds (shared pairs) from the central atom to each surrounding atom.
The bonds are represented as lines; each line represents two electrons.
The number of lines should be equal to the number of shared pairs
calculated in step 3, which in this case is four. Since hydrogen follows the
duet rule, it only prefers one bond. The fourth bond can be drawn to either
one of the oxygen atoms.
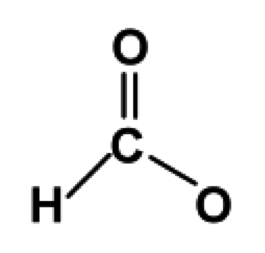
Figure 2
7
Draw lone pairs on each of the non-hydrogen atoms. A lone pair is
represented as two dots; each dot represents an electron. Each non-hydrogen
atom prefers eight electrons in the vicinity of the atom. If an atom
has 1 bond, it requires 3 lone pairs. If an atom has 2 bonds, it requires 2
lone pairs. If an atom has 3 bonds, it requires 1 lone pair. If an atom has 4
bonds, do not add any additional lone pairs. In our example, C requires no
lone pairs, one oxygen requires 3 lone pairs and one oxygen requires 2
lone pairs.
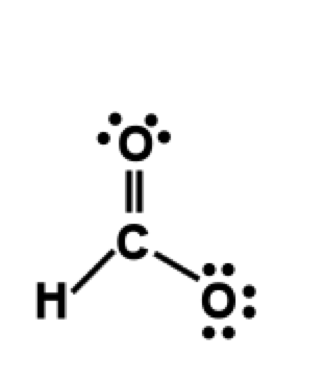
Figure 3
8
Calculate the formal charge (FC) on each atom. For an atom:
where SP is the number of shared pairs on the atom and LP is the number
of lone pairs on the atom. For molecules the sum of the formal charges of
all the atoms must be zero; for an ion, the sum will be the ionic charge. To
choose the more favorable of two Lewis structures, the one with the lowest
FC on each individual atom would be the most likely candidate. Remember
that the more electronegative atom will generally prefer the negative formal
charge. The formal charge for each atom on the formate ion is shown
below. Note how the sum of the formal charges (0 + 0 + 0 + –1) adds up to the –1 charge on the formate ion.
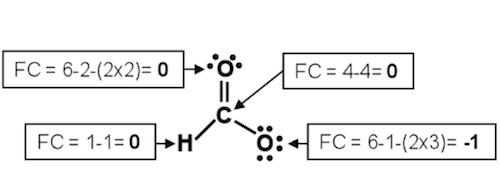
Figure 4
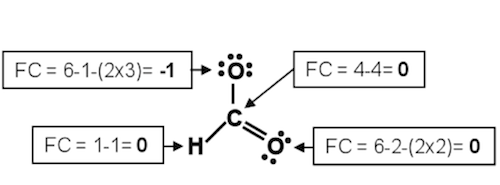
Figure 5
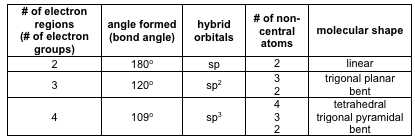
Figure 6
Equipment
- 1 Chem-Tutor model kit
- 1 computer with internet browser
- 1 Molecular Geometry In-Lab assignment in WebAssign
About the model kit
The Chem-Tutor model kit was designed by Professor Samuel G. Levine for use by his organic chemistry students at North Carolina State University. Atoms: Atoms are represented by jacks that have one, two, three or four pegs coming out. Each peg represents an electron region (or electron group). The electron regions (groups) can be single bonds, double bonds, triple bonds or lone pairs. In general, black jacks are used for carbon, blue for nitrogen, red for oxygen, white for hydrogen and yellow for sulfur. More important than color is the number of pegs. The type of atom/jack to use is determined by the Lewis structure of the molecule. Bonds: Green bonds can be used for single, double or triple bonds. Do not bend the green bonds to force a molecule into a particular shape. Although you are strong enough to bend these bonds, it takes energy for atoms and molecules to bend away from the predicted angles and shapes. The molecules prefer to exist in the lowest energy configurations ("straight" green bonds). Black bonds between two carbon atoms can be used for carbon-carbon double bonds. You will notice that the pegs on each carbon define a plane, and that the two carbons are coplanar. The atoms at the ends of the bond are fixed in place. DO NOT attempt to twist this bond. White bonds are made out of rubberized plastic, and are the only ones that are meant to bend. They are only used for special "strained" structures that you should not encounter in this lab.About the computer modeling software
The molecules in your WebAssign In-Lab are viewed with jmol, a javascript plug-in. The computer files viewed with jmol contain real data from measurements on these molecules. Often we find that molecules follow what is predicted from textbooks. Occasionally, they do not. Do not be surprised if what you observe is sometimes different than what you might predict! Rotating a molecule: A molecule in the viewing area can be rotated in all directions. This can be achieved by holding down the left mouse button while moving the mouse. After a few tries at this, you should be able to manipulate the molecule any way that you want. To determine a bond length: Double click on one of the atoms in the bond. The cursor will change to a "+". Then double click on the other atom in the bond. The measurement will be displayed in nanometers (nm). To make the measurement disappear, re-measure the bond in the same way. To determine a bond angle: Double click on one of the atoms of the angle. The cursor will change to a "+". Then single click on the vertex of the angle. And last, double click on the third atom in the angle. The measurement will be displayed in degrees. To make the measurement disappear, re-measure the angle in the same way.Reagents
No chemicals will be used in this experiment.Safety
None of the materials being used in this experiment present a safety hazard. However, the work is being done in a laboratory and the usual rules about eye protection and proper clothing apply.Waste Disposal
Since no chemicals are being used in this experiment, there will not be any waste for disposal.Lab Procedure
You will notice the format of this lab experiment is different than other experiments. You will build a series of models and investigate them on the computer. Questions to help you with your observations are intermingled with the procedure. Please answer the questions in your lab manual along with any other observations you make while you are building the structures.-
•Launch Internet Explorer.
-
•Open one partner's Molecular Geometry In-Lab in WebAssign.
Part A: Exploring Simple Structures
For each of the following molecules,-
•Review the correct, complete Lewis structure(s), including any resonance structures and any formal charges that you drew in your Prelab assignment.
-
•Build the molecule with the model set.
-
•Look at the molecule in your In-Lab assignment on WebAssign.
-
•Fill in the table and answer the questions below.
1
Two Electron Regions
-
Dinitrogen Oxide (N2O) Hint: Nitrogen is the central atom.
2
Three Electron Regions
-
aSulfur Dioxide (SO2)
-
bFormaldehyde (CH2O)
3
Four Electron Regions
-
aWater (H2O)
-
bAmmonia (NH3)
-
cMethane (CH4)
Part B: Bond Order vs Bond Length
For each of the following molecules,-
•Review the correct, complete Lewis structure(s), including any resonance structures and any formal charges that you drew in your Prelab assignment.
-
•Build the molecule with the model set.
-
•Look at the molecule in your In-Lab assignment on WebAssign.
-
•Fill in the table and answer the questions below.
1
Ethane (C2H6)
2
Ethene (C2H4)
3
Ethyne (C2H2)
Part C: Resonance Structures
Draw the Lewis structures for Part C on your lab worksheet. For each of the following molecules,-
•Review the correct, complete Lewis structure(s), including any resonance structures and any formal charges that you drew in your Prelab assignment.
-
•Build the molecule with the model set.
-
•Look at the molecule in your In-Lab assignment on WebAssign.
-
•Fill in the table and answer the questions below.
1
Benzene (C6H6) Hint: Place the carbons in a hexagon.
2
Carbonate Ion (CO32–)
3
Thiocyanate Ion (SCN–) Hint: Carbon is the central atom.
-
•When you are done, clean up any model set structures and leave the sets as you found them when you arrived in the lab.
-
•Before leaving, go to a computer in the laboratory and enter your results in the In-Lab assignment. If all results are scored as correct, log out. If not all results are correct, try to find the error or consult with your lab instructor. When all results are correct, note them and log out of WebAssign. The In-Lab assignment must be completed by the end of the lab period. If additional time is required, please consult with your lab instructor.