
Lab 10 - Acid-Base Studies
Purpose
To measure pH's in a variety of solutions and mixtures and to account for the results obtained.
Goals
-
1
To learn to use pH paper and a pH meter to measure the pH of a given solution.
-
2
To become familiar with the pH scale.
-
3
To observe pH changes produced upon addition of acid or base to a solution.
Introduction
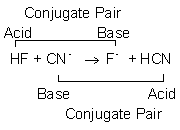
Many substances can be classified as acids or bases. There are three definitions used to describe acids and bases, but we consider only the Brønsted definition here. In this theory, an acid is a proton (H+) donor and acids can usually be recognized because protons that can be transferred are written first in the chemical formula. For example, acetic acid has the formula HC2H3O2. Although it contains four protons, only one is acidic. A base is a proton (H+) acceptor. Protons have positive charge, so their acceptors usually have negative charge, i.e., most anions are bases. NH3 is the most common base that is not an anion. A Brønsted acid-base reaction is the transfer of a proton from the acid to the base to form their conjugate acid-base pairs. Conjugate acid-base pairs differ by exactly one proton. Thus, the conjugate base of an acid is obtained by removing one H+, so the conjugate base of HF is the F– ion. The conjugate acid of a base is obtained by adding one H+ to the base, so the conjugate acid of CN– is HCN. Brønsted acid-base reactions contain two conjugate acid-base pairs and nothing else.
The acidity of a Brønsted acid is a measure of the extent to which the acid reacts with the weak base H2O to produce its conjugate base and H3O+ ions, the conjugate acid of water. The greater the extent of this reaction, the larger the equilibrium constant is for the reaction. This equilibrium constant is defined as the acid dissociation constant or Ka of the acid. The larger the Ka, the stronger the acid is. The general form of the acid dissociation reaction and Ka are shown below in equation 1.
( 1 )
HA + H2O 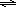 A− + H3O− A− + H3O− | | Ka = |
The basicity or alkalinity of a Brønsted base is a measure of the extent to which a Brønsted base reacts with water to produce its conjugate acid and OH– ions, the conjugate base of water. The equilibrium constant for the reaction of a base with water is given the symbol Kb. The general from for this chemical reaction and Kb are shown below in equation 2.
( 2 )
A− + H2O 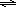 HA + OH− HA + OH− | | Kb = |
The acidity of an aqueous solution is therefore measured by the concentration of H3O+, and the basicity by the concentration of OH–. Most solutions we deal with are aqueous solutions, and these concentrations are important characteristics. We have seen that water behaves both as an acid (Reaction 1) and a base (Reaction 2), so it should not be surprising that it can react with itself.
( 3 )
H2O + H2O 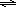 H3O+ + OH− H3O+ + OH− | | Kw = [H3O+][OH−] |
Hydronium and hydroxide ion concentrations can be very small. For example, the Kw = 1.0 x 10–14 at 298K, so in pure water at 298 K, [H3O+] = [OH–] = 1.0 x 10–7 M. Negative exponents are avoided by defining the pH.
The pH of pure water is –log(1.0 x 10–7) = 7.0. Solutions in which [H3O+] = [OH–] are said to be neutral, so neutral solutions have a pH of 7.0 at 298 K. Solutions in which [H3O+] > [OH–] are acidic and pH < 7. Solutions in which [OH–] > [H3O+] are basic or alkaline and pH > 7. Thus, the acidity or basicity of an aqueous solution can be determined from its pH. Equation 4 can be solved for the hydronium ion concentration to obtain Equation 5.
Thus, a pH of 2.2 implies that [H3O+] = 10–pH = 10–2.2 = 0.006 M.
The acidity or basicity of a solution can be determined by measuring its pH or with an indicator. pH meters are electrochemical cells whose voltage is pH sensitive. When the electrode from the meter is placed in a solution, a voltage is developed between the meter and the solution. The voltage is converted to a pH that can be read directly. An acid-base indicator is a weak acid whose conjugate base is a different color. When the indicator is placed in an acidic solution, it is converted to the acid form and takes that color, but when it is placed in a basic solution, it is converted to the base and takes on the color of the base.
pH paper is a strip of paper with an indicator on it. The color of the indicator changes with pH, so the approximate pH of a solution can be determined by placing a small amount of the solution on the paper.
In Part A of this lab, you will perform a 3-point calibration on a pH meter. In Part B, you will use pH meters to determine the pH of several acid-base solutions commonly found in a chemistry laboratory to become familiar with the pH scale. In Part C, you will use pH paper to determine whether several common household chemicals are acidic, basic, or neutral. In Part D, you will use a pH meter to follow the pH change of an acidic solution as a basic solution is added to it.
Equipment
-
1 30 mL beaker
-
1 10 mL graduated cylinder
-
1 glass stir rod
-
7 medium test tubes
-
1 test tube rack
-
1 pH electrode in pH 7.00 buffer
-
1 MicroLab interface
-
1 MicroLab pH measurement instruction sheet
-
1 pH paper
-
1 plastic work surface
-
1 deionized water squirt bottle
Reagents
-
~25 mL 0.010 M HCl
-
~10 mL 0.0010 M HCl
-
~10 mL 0.00010 M HCl
-
~10 mL 0.010 M HC2H3O2
-
~25 mL 0.010 M NaOH
-
~10 mL 0.0010 M NaOH
-
~10 mL 0.010 M NH3
-
~15 mL pH 4.00 buffer
-
~15 mL pH 7.00 buffer
-
~15 mL pH 10.00 buffer
-
~2 drops vinegar
-
~2 drops bleach
-
~2 drops ammonia
-
~2 drops vitamin C
-
~2 drops lemon juice
-
~2 drops baking soda
-
~2 drops dishwasher detergent
-
~2 drops carbonated water
-
~2 drops baking powder
Safety
HCl, NaOH, HC2H3O2, and NH3 are corrosive. They can attack the skin and cause permanent damage to the eyes. If any of these solutions splash into your eyes, use the eyewash immediately. Hold your eyes open and flush with water. If contact with skin or clothing occurs, flush the affected area with water. Have your lab partner notify your instructor about the spill.
HCl, HC2H3O2, and NH3 solutions give off highly irritating vapors. Do not inhale them. Work with concentrated solutions under the hood so vapors do not build up in the lab. If you do inhale enough vapor to have a problem, move to fresh air. Have your lab partner notify your instructor about the accident.
Acid-base reactions are highly exothermic. They can cause water to boil and splash hot, corrosive solution out of the vessel in which they were mixed. Do not directly combine solutions with concentrations greater than 0.1 M. Use caution when pouring solutions or disposing of them.
Waste Disposal
All solutions can be flushed down the sink with plenty of water. When disposing of concentrated solutions, pour them slowly while the water is running.
Prior to Class
Please complete WebAssign prelab assignment. Check your WebAssign Account for due dates. Students who do not complete the WebAssign prelab are required to bring and hand in the prelab worksheet.
Lab Procedure
Please print the worksheet for this lab. You will need this sheet to record your data.

In this experiment, you will be using pH meters. They have electrodes with a thin glass bulb at the tip. They break easily and are costly to replace. Be careful not to shove the electrode into the bottom of a test tube or drop the electrode. There is a protective guard around the tip, which should remain in place at all times. The guard will not protect against careless treatment. Please use extreme care when using this equipment. When the pH electrode is not in use, it should be capped with the pH 7 buffer solution.
Part A: Calibration of a pH Meter
-
1
Open the MicroLab program.
-
2
Make sure the pH electrode is plugged into the interface.
-
3
Calibrate the pH electrode using the MicroLab instructions provided in the lab. The calibration stands for the pH electrode will be pH = 4.00 (red),
pH = 7.00 (yellow), and pH = 10.00 (blue) buffer solutions. Use about 15 mL of each in 30 mL beakers.
-
4
After the calibration is complete, configure the MicroLab program to collect data as described in the instructions provided in the lab.
Part B: pH Measurements of Some Common Acid and Base Solutions
-
1
Number seven test tubes 1–7.
-
2
Fill each test tube ~1/4–1/2 full with solutions 1–7 listed in Data Table A.
-
3
Use a pH meter to measure the pH of each solution and record them in Data Table A. Rinse the pH meter thoroughly with deionized water between each measurement.
-
4
Discard the solutions and rinse each of the test tubes.
Part C: Acidity and Basicity of Some Household Chemicals
-
1
Place ten small strips of pH paper on the plastic work surface provided.
-
2
Put one or two drops of each of the solutions listed in Data Table B on a separate piece of paper. Because ammonia vapors will react with the pH paper, add ammonia (NH3) last! The bleach will oxidize the pH paper quickly; be sure to observe the initial color change of the pH paper.
-
3
Observe and record your results in Data Table B.
Part D: Acid-Base Reactions
-
1
Measure 10.0 mL of 0.010 M HCl in a 10 mL graduated cylinder and place in a clean 30 mL beaker.
-
2
Use a pH meter to measure the pH. Record this value in Data Table C as 0.0 mL NaOH.
-
3
Rinse the graduated cylinder used in step 1 thoroughly and dry.
-
4
Measure 3.0 mL of 0.010 M NaOH and add it carefully to the HCl solution in the beaker and stir. Record the pH of the new solution in Data Table C as 3.0 mL NaOH.
-
5
Add an additional 3.0 mL of 0.010 M NaOH to the beaker, stir, and record the pH in Data Table C as 6.0 mL NaOH. The total volume in the beaker should now be 16 mL.
-
6
Add a final 6.0 mL of 0.010 M NaOH to the beaker and record the pH in Data Table C as 12.0 mL NaOH. The total volume should now be 22 mL.
-
7
Turn off the pH probe.
-
8
Discard the solution, wash and dry all your glassware and return it to the set-up area where you found it.
-
9
Before leaving, go to a computer in the laboratory and enter your results in the In-Lab assignment. If all results are scored as correct, log out. If not all results are correct, try to find the error or consult with your lab instructor. When all results are correct, note them and log out of WebAssign. The In-Lab assignment must be completed by the end of the lab period. If additional time is required, please consult with your lab instructor.

 A− + H3O−
A− + H3O−