Lab 1 - Density Determinations and Various Methods to Measure Volume
Goal and Overview
This lab provides an introduction to the concept and applications of density measurements. The densities of brass and aluminum will be calculated from mass and volume measurements. To illustrate the effects of precision on data, volumes will be determined by three different methods: geometrically (measuring lengths); water displacement; and pycnometry. The composition of a mixed brass-aluminum cylinder and the volume of empty space within a hollow cylinder will also be found.Objectives of the Data Analysis
-
•Determine volume by three different methods
-
•Use measured volumes and masses to calculate densities
-
•Use the relationship between mass, volume, and density to find desired unknown quantities
-
•Evaluate results using error analysis
Suggested Review and External Reading
-
•Data analysis introduction, textbook information on density
Introduction
The density, ρ, of an object is defined as the ratio of its mass to its volume. Density can be useful in identifying substances. It is also a convenient property because it provides a link (or conversion factor) between the mass and the volume of a substance.( 1 )
ρ = m/V
(i)
volume by geometry
(ii)
volume by water displacement
(iii)
volume by pycnometry (mass-based)
(i)
the volume of a void inside a hollow cylinder; and,
(ii)
the percent composition of a mixed-metal cylinder.
Volume by geometry
A cylinder is a standard geometric form. In this case, you can measure the dimensions of the cylinder and apply the formula to calculate its volume.( 2a )
V = π
l =
d 2 l where d = diameter and l = length.
 |
| d |
| 2 |
 | 2 |
| π |
| 4 |

Figure 1
( 2b )
ρ =
=
| m |
| V |
| 4m |
| πd 2 l |
( 2c )
σ ρ = ± ρ
+
+
+
 |
 |
| σ d |
| d |
 |
 |
| σ d |
| d |
 |
 |
| σ l |
| l |
 |
 |
| σ m |
| m |
 |
 |
Volume by water displacement
For less defined shapes, volume can be determined by water displacement. Volumes of liquids such as water can be readily measured in a graduated cylinder. To use the water displacement method, an object (in this case, a small metal cylinder) is inserted into a graduated cylinder partially filled with water. The object's volume occupies space, displacing liquid and raising the water level. The difference between the two volumes, before and after the object was inserted, is the object's volume.
Figure 2
( 3a )
Vcyl = Vfinal − Vinitial = Vwater + cyl − Vwater
( 3b )
σ Vcyl = σ Vfinal + σ Vinitial
( 3c )
σ ρ = ± ρ
+
 |
 |
| σ V |
| V |
 |
 |
| σ m |
| m |
 |
 |
Volume by pycnometry
Pycnometry is a technique that uses the density relationship between volume and mass, and the vessel used is called a pycnometer . To perform pycnometry measurements, the mass of the cylinder and the mass of a flask filled with water to a mark (A, Fig. 3) are recorded. The cylinder is then inserted into the flask. Water is displaced when the cylinder is inserted. The volume of water displaced is removed by pipet, thereby restoring the water level to the mark (B). The combined mass of the flask, remaining water, and cylinder is then measured.
Figure 3
( 4a )
| massA + masscylinder = massB + massdisplaced water |
| massdisplaced water = massA + masscylinder − massB |
( 4b )
Vdisplacedwater = Vcylinder = massdisplaced water / densitywater
( 4c )
 |
 |
mA,trial1 + mA, trial2 + mA, trial3 +  |
| # trials |
( 4d )
σmA = ±
 |
|
( 4e )
σmwater = σmA + σmB + σmcyl = σmA + σmA + σmcyl
( 4f )
σρ = ± ρ
+
 |
 |
| σV |
| V |
 |
 |
| σm |
| m |
 |
 |
Volume of a void inside a hollow cylinder
A hollow cylinder has an empty space inside.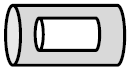
Figure 4
( 5a )
Vcyl = Vmetal + Vvoid → Vvoid = Vcyl - Vmetal
( 5b )
Vmetal =
No error propagation is required
| mcyl |
| ρmetal |
Percent composition of a mixed cylinder
The total mass of the cylinder, mcyl, is the sum of the mass of Al and brass (mAl + mbrass). In terms of fractional composition, this would be Xmcyl and (1 - X)mcyl, respectively, where X is the Al fraction and (1-X) is the brass fraction (the remainder). The cylinder volume is determined by pycnometry and is the sum of the volumes of the two metals:( 6a )
Vcyl = VAl + Vbrass
( 6b )
Vcyl =
+
| mAl |
| ρAl |
| mbrass |
| ρmass |
( 6c )
Vcyl =
+
| X mcyl |
| ρAl |
| (1−X)mcyl |
| ρbrass |
( 6d )
| 1 |
| ρcyl |
| X |
| ρAl |
| (1 − X) |
| ρbrass |
( 6e )
| 1 |
| ρcyl |
 |
| 1 |
| ρAl |
| 1 |
| ρbrass |
 |
| 1 |
| ρbrass |
( 6f )
X =
| ||||||
|
a
Calculate each fraction in the equation, then the differences, and then the final ratio.
b
Use the densities of brass and aluminum determined experimentally.
c
Find X, and use X to determine the mass fraction of brass in the mixed cylinder, 1 - X.
X has a range of possible values from zero to one (0 - 100%). If your mixed cylinder's density is between that of aluminum and of brass, you should calculate a percent of aluminum that makes sense. For example, if the mixed cylinder has a density
near that of Al, X should be near one.
Equipment List
- cylinders: brass, aluminum, mixed brass/aluminum, and hollow
- Vernier caliper
- 50 mL Erlenmeyer flask, 100 mL graduated cylinder, 400 mL beaker
- lab marker
- Pasteur pipet
- thermometer
Procedure
NOTE: IF YOU WORK WITH ANOTHER SET OF PARTNERS, MAKE SURE YOU RECORD ALL DATA. YOU WILL NOT BE ABLE TO COMPLETE THE DATA ANALYSIS IF YOUR DATA TABLES ARE INCOMPLETE. ALSO CHECK THAT DATA MAKES SENSE. Parts 1-3. Density of aluminum and brass cylinders using three different methods of volume measurementPart 0: Measure metal cylinder masses.
1
Obtain four cylinders - brass, aluminum (solid cylinders marked S), hollow (marked H), mixed brass/aluminum (marked P for "plugged"). Return cylinders to the stockroom at the end of lab.
2
Record the cylinders' numbers.
3
Record the masses of the cylinders on the analytical balance to the 0.0001 g (the uncertainities in your cylinders' masses are ±0.0001 g). You will use these masses throughout the experiment.
Part 1: Volume by Geometry
1
Measure the diameter and length of each cylinder using the Vernier calipers. Your TA will help you if you need it. Record the values to the 0.01 cm (each measurement is ±0.01 cm).
2
Determine the density of the cylinder. Find the uncertainity using error propagation.
Part 2: Volume by Displacement
1
Put enough water to cover the metal cylinder into a 100-mL graduated cylinder and record the volume. The graduated cylinder is not very precise; readings will be ±0.5 mL (the digit in the tenths place will either be a 5 or a 0).
2
Carefully slide the metal cylinder down the side of the graduated cylinder into the water. Tossing it in can break the bottom of the graduated cylinder.
3
With the metal cylinder completely submerged, record the new volume reading (to ±0.5 mL).
4
Determine the volume of the metal cylinder. Calculate the uncertainty in your volume using error propagation.
5
Determine the density of the cylinder. Calculate the uncertainty using error propagation.
Part 3: Volume by Pycnometry
1
Fill a 400 mL beaker with water and measure its temperature. Use this water throughout the experiment. Assume that the density of water makes a negligible contribution to the overall uncertainty in the values calculated.
2
Make your pycnometer.
-
aDraw a ring midway up the neck of a 50 mL Erlenmeyer flask with a waterproof marker or wax crayon, as shown below.
-
bInvert the flask on the table; hold marker on top of something solid; and, rotate the flask while marking the neck at a constant height.

Figure 5
3
Calibrate your pycnometer. How well can you adjust the water's meniscus to the top of the line drawn? Precise filling to that mark increases reproducibility (and data quality). Practice with the pycnometer before making measurements. Your TA will demonstrate.
The pycnometer filled with water to the mark is called 'A'.
-
aUse a disposable pipet to add and remove drops of water to adjust the meniscus to the top of the line.
-
bRecord the mass of the flask and water. No drops should appear on the neck of the flask above the water line.
-
cPour out the water into your 400-mL beaker; refill to the mark; reweigh.
-
dRepeat step c until you have three similar values for the mass of 'A'.
-
eDetermine the average mA and its standard deviation (σmA). The standard deviation, σmA, reflects your ability to reproducibly fill the pycnometer to the same place every time you use it; σmA is the uncertainity of the pycnometer and should be read with the average mass of 'A' as well as the mass of 'B' (parts 3, 4, and 5).
-
fIf you share data with another set of students, make sure to record their calibration data as well. You must use the correct calibration result with the appropriate data. In your lab notebook, label which cylinders go with which pycnometer calibration.
4
Indirectly measure the mass of water displaced by your solid cylinders. The pycnometer containing the metal cylinder with water filled to the mark is called 'B'.
-
aCarefully insert a metal cylinder, fill with water to the mark, and record the mass (the flask with water and cylinder).
-
bRepeat filling and weighing several times until the data appears reproducible.
-
cCalculate the mass of the water removed. Convert this mass to volume by dividing by the density of water (use a precise value, specific to the water's temperature). This volume equals the volume of the metal cylinder.
- Calculate the uncertainty in the mass of water removed using error propagation. Convert this mass to volume units by dividing by the density of water (use a precise value, specific to the water's temperature). This value equals the uncertainity in the volume of the metal cylinder.
-
eRepeat with the other cylinders as instructed.
5
Determine the density of each cylinder. Include the uncertainties.
Part 4: Determine Void Volume in a Hollow Cylinder by Pycnometry
1
For the hollow cylinder, record identity of metal of the hollow cylinder (either aluminum or brass). You recorded its mass at the beginning of the experiment.
2
Insert the cylinder into the pycnometer; remove the water above the line, and record the new mass.
3
Determine the volume of the cylinder and calculate the volume of the void. No error propagation.
Part 5: Mass Fraction of Al and Brass Determination for Mixed-metal Cylinder by Pycnometry
1
You recorded its mass of the mixed metal cylinder at the beginning of the experiment.
2
Insert the mixed Al/brass cylinder into the pycnometer, remove the water above the line, and record the mass.
3
Determine the density of the cylinder and the mass fractions of Al and of brass (X and 1-X, respectively). No error propagation.
Reporting Results
Complete your lab summary or write a report (as instructed).Results / Sample Calculations
- Masses and volumes for solid cylinders by each method
- %error relative to literature values (ρAl = 2.70 g/cm3; ρAl = 8.44 g/cm3, depending on the alloy's composition)
- Void volume
- Mass fraction
- Error analysis for parts 1-3
Discussion
- What you found out (refer to results tables) and how for all 5 parts
- What were the major experimental sources of error?
- Compare the three methods used to determine volume - which method was more accurate and why? Which was most precise?
- What could be done to improve the precision in any or all of the methods?
- How does the instrument error compare to standard deviation error?
