Example Abstracts for a General Chemistry Lab
Title
Gravimetric Determination of the Solubility Product Constant for Lead (II) Chloride, PbCl2Introduction
In this experiment, the equilibrium exhibited by slightly soluble ionic compounds in water is explored. Most ionic compounds, even those called "soluble", have a limited solubility in water. If more than this amount is added, some solid will remain undissolved. In a saturated solution at a particular temperature, equilibrium exists between the undissolved and dissolved solid. Slightly soluble ionic compounds are often called "insoluble" because they have a relatively low solubility (little dissolves before equilibrium is reached). Lead (II) chloride, the insoluble ionic compound used, is assumed to dissociate according to equation 1.( 1 )
PbCl2(s) 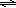 Pb2+(aq) + 2 Cl−(aq)
Pb2+(aq) + 2 Cl−(aq)
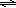 Pb2+(aq) + 2 Cl−(aq)
Pb2+(aq) + 2 Cl−(aq) Ksp,
the equilibrium constant for the dissociation reaction, is written according to equation 2.
( 2 )
Ksp = [Pb2+][Cl−]2
Ksp
is then calculated using Eq. 2Ksp = [Pb2+][Cl−]2
. Since PbCl2 is "insoluble", Ksp
should be very small (<<1). This reflects the fact that the concentration of the dissolved ions, Pb2+ and Cl–, is very low.
Abstract
Instructions
Rate the following abstracts from 1 to 5.- 1 = beginning, 2 = developing, 3 = adequate, 4 = accomplished, 5 = exemplary
Sample Abstracts
A
The Ksp
for PbCl2 dissociation was found. Three trials were performed using about 0.770 g PbCl2 each time. One trial was performed in 25.00 mL pure water; one trial was performed in 25.00 mL 0.10 M NaCl; and, one trial was performed in 25.00 mL 0.10 M Pb(NO3)2 so the effect of additional dissolved ions could be assessed. Ksp
of PbCl2 was found to be 1.59 × 10–5. Even though it was hard to measure the Pb2+ and Cl– concentrations, the results were pretty good.
B
Equilibrium dissociation constants that compare favorably with literature values can be obtained by the gravimetric method used in this work. The solubility product constant, Ksp,
of lead (II) chloride was found to be 1.59 × 10–5 ± 6.00 × 10–7 at 298 K, which is within 1% of the accepted value. Primary sources of error can be minimized if the work is performed carefully.
C
We calculated Ksp
for PbCl2 which is an ionic compound that doesn't dissolve in water too much but does a little bit depending on factors like temperature and other things. We had to do three tests with solid PbCl2 and pure water or 0.10 M NaCl or 0.10 M Pb(NO3)2
and then figure out how much Pb2+ and Cl– were in the solution part. We got a Ksp
that was close to the value our TA said was right.
D
PbCl2, an "insoluble" ionic compound, has a low solubility product constant (Ksp
) of 1.6 × 10–5 at 25°C. Using gravimetric analysis, the experimentally determined Ksp
of PbCl2 was found to be 1.59 × 10–5 ± 6.00 × 10–7. The small percent difference between the expected and observed Ksp
values indicates that this method of analysis is a valid and accurate way of determining the extent of dissociation of slightly soluble ionic compounds in water. Problems such as precipitate loss and/or contamination during filtration can introduce error if care is not taken during the experiments.
