Experiment 8 - Amide Preparation
Objective
In this experiment, the amide, 2-(N-acetylamino)benzoic acid (N-Acetylanthranilic acid) will be prepared by the reaction of 2-aminobenzoic acid (anthranilic acid) with acetic anhydride.Introduction
Amides are another one of the many functional groups encountered in the study of Organic Chemistry. The amide is identified by having a carbonyl unit (C=O) bound to a Nitrogen unit (NR2, R can be H).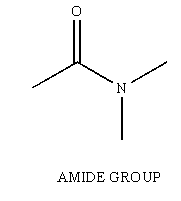
Figure 1
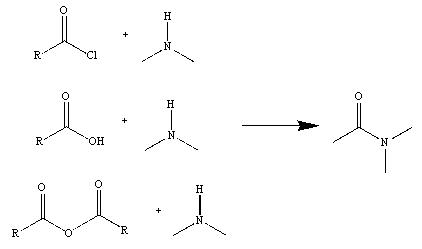
Figure 2
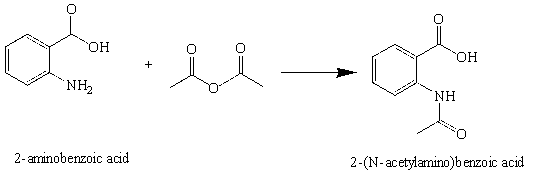
Figure 3
Pre-Lab
Complete the pre-lab assignment in WebAssign.Procedure
Make sure that the reaction is performed under the sink hoods (i.e., not out in the middle of the bench top). Acetic anhydride is a lachrymator (what does that mean?). In a 25 mL Erlenmeyer flask, place 1 g of anthranilic acid and 3-4 mL of acetic anhydride. Warm the mixture to boiling (gently) on the hot plate. Any solid should dissolve. Heat for a period of 15 minutes. Allow to cool, add 2 mL of water, and heat again to boiling. Slowly let the reaction mixture cool to room temperature (i.e., no ice bath, or cold tap water). The crystalline product should form as the mixture cools. Isolate the product by vacuum filtration and wash with a small amount of cold water. Determine the yield, percentage yield, and melting point of the product. The filtrate may be neutralized with aqueous NaHCO3, diluted with water, and flushed down the drain.In-Lab Questions
Download and print the following worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of anthranilic acid used ___________________ g, ___________________ mol
- Question 2: Theoretical Yield of product _____________________
- Question 3: Actual Yield of product _____________________
- Question 4: Percentage Yield _____________________
- Question 5: Melting Point ___________________ (observed), ___________________ (actual)
- Question 6: Record your calculations.