Experiment 7 - Preparation of 1,4-diphenyl-1,3-butadiene
Objective
To provide experience with the "Wittig Reaction", one of the most versatile reactions available for the synthesis of an alkene.Introduction
The carbon-carbon double bond, the "Functional Group" of all alkenes, is a very common functionality. It can be prepared in a variety of ways, many of which involve an "Elimination" reaction. For example, alcohols may be dehydrated under the influence of strong acid, or alkyl halides may be dehydrohalogenated in the presence of strong base. Both of these reactions have the disadvantage of employing harsh reaction conditions. In addition, the former reaction frequently results in skeletal rearrangement. A very general procedure for connecting two molecular fragments to make a new and larger molecule was announced in 1954 by the German Chemist Georg Wittig. For this discovery, Wittig was awarded the Nobel Prize in Chemistry in 1979.
Figure 1

Figure 2

Figure 3
Equation
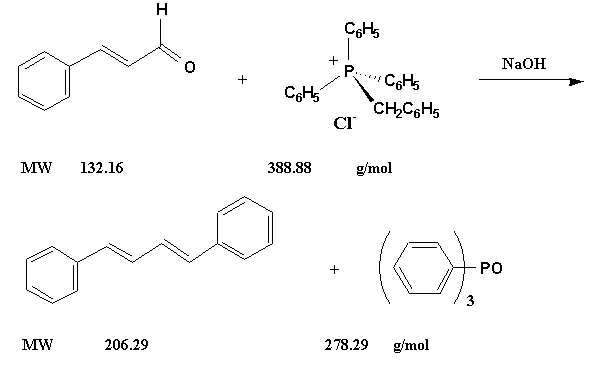
Figure 4
Pre-Lab
Complete the pre-lab assignment in WebAssign.Caution:
Sodium hydroxide is highly corrosive. Rinse any spills with large amounts of cold water. Methylene chloride, or dichloromethane (CH2Cl2), a common component of paint and varnish remover, is a very volatile, toxic compound. Avoid breathing the vapors. Immediately seek fresh air if you should breathe a large amount of the vapor and feel light headed.
Sodium hydroxide is highly corrosive. Rinse any spills with large amounts of cold water. Methylene chloride, or dichloromethane (CH2Cl2), a common component of paint and varnish remover, is a very volatile, toxic compound. Avoid breathing the vapors. Immediately seek fresh air if you should breathe a large amount of the vapor and feel light headed.
Procedure
[adapted from: S. W. Breuer, J. Chem. Educ. 1991, 68, A58-A60.]
Use only clean and DRY glassware!
Caution:
Concentrated sodium hydroxide (NaOH) is very corrosive. It will burn the skin. Wash any area that contacts the solution with copious amounts of cold water.
Concentrated sodium hydroxide (NaOH) is very corrosive. It will burn the skin. Wash any area that contacts the solution with copious amounts of cold water.
Waste Disposal
Alcohol from the recrystallization may be washed down the drain with tap water. The aqueous solution in the first 6" test tube should be poured into the waste bottle labeled "AQUEOUS WITTIG WASTE". Any solid product should be added to the jar marked "STUDENT PREPARATION - 1,4-DIPHENYL-1,3-BUTADIENE".In-Lab Questions
Download and print the worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of cinnamaldehyde ____________________ g, ____________________ mol
- Question 2: Amount of Wittig reagent ____________________ g, ____________________ mol
- Question 3: Theoretical Yield of product ____________________ mol, ____________________ g
- Question 4: Actual Yield ______________________
- Question 5: Percentage Yield ______________________
- Question 6: Melting Point ______________________ (observed), ______________________ (reported)
- Question 7: Record your calculations.