Experiment 4 - Oxidation of Acetophenone
Objective
-
•to demonstrate a simple application of the "Iodoform" reaction
-
•to illustrate that common household chemicals can be important reagents
-
•to utilize simple test tubes to run a reaction
Introduction
The haloform reaction is characteristic for methylketones as well as for alcohols (e.g. ethanol, 2-propanol), that can be oxidized to methyl carbonyl compounds. The iodoform test is commonly used as a test for the CH3—CO group. The group to which the CH3—CO group is attached can be aryl, alkyl and hydrogen. In this experiment you will explore a variant of the haloform reaction, using bleach as the oxidizing agent.Mechanism of the Classic Iodoform Reaction
- Step 1: First, an acid-base reaction. Hydroxide functions as a base and removes the acidic α-hydrogen, giving the enolate.
- Step 2: The nucleophilic enolate reacts with the iodine giving the halogenated ketone and an iodide ion.
- Step 3: Steps 1 and 2 repeat twice more yielding the trihalogenated ketone.
-
Step 4: The hydroxide now reacts as a nucleophile at the electrophilic carbonyl carbon, with the C
 O becoming a C—O single bond and the oxygen is now anionic.
O becoming a C—O single bond and the oxygen is now anionic.
-
Step 5: Reform the favorable C
 O and displace a leaving group, the trihalomethyl system which is stabilized by the 3 halogens. This gives the carboxylic acid.
O and displace a leaving group, the trihalomethyl system which is stabilized by the 3 halogens. This gives the carboxylic acid.
- Step 6: An acid-base reaction. The trihalomethyl anion is protonated by the carboxylic acid, giving the carboxylate and the haloform (trihalomethane).
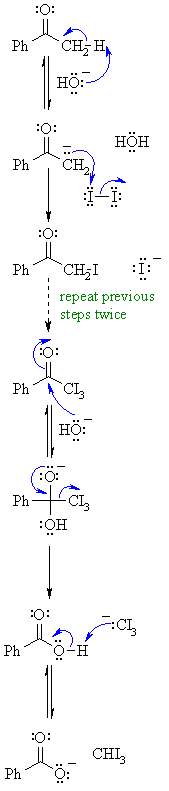
Figure 1
Pre-Lab
Complete the pre-lab assignment in WebAssign.Equation
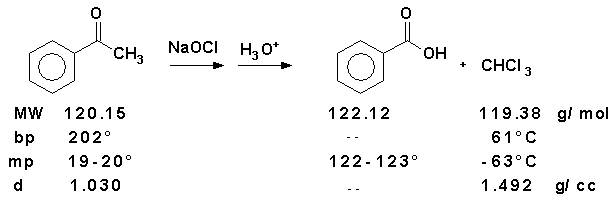
Figure 2
Procedure
Using a dispensing pipet, carefully add 180 µL of acetophenone to a clean 6" Test Tube. Measure 6.3 mL of household bleach (e.g., "CLOROX"TM) in a 10 mL graduated cylinder and carefully add it to the same test tube, followed by 0.5 mL of 10% NaOH. Heat the reaction mixture in a water bath (~75°C) for 20 minutes. During the heating, frequently shake the test tube to ensure mixing of the reagents. While the solution is being heated, weigh out about 45.0 mg of sodium sulfite. After the solution has reacted for 20 min., carefully add the sodium sulfite to destroy any unreacted bleach (NaOCl). Shake this mixture for 5 min. Extract the mixture with a 1.5 mL portion of diethyl ether using your calibrated Pasteur pipet to transfer the ether into and out of the test tube.Caution:
Diethyl ether is a highly flammable solvent!
Diethyl ether is a highly flammable solvent!
Waste Disposal
The ether from the extraction may be carefully evaporated in the hood under a stream of nitrogen. The aqueous washings may be adjusted to a pH between 5.5 and 10.5 by the addition of 5% NaHCO3 and then washed down the drain in a stream of cool water.In-Lab Questions
Download and print the worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of acetophenone used ____________ mL, ______________ g, _____________ mol
- Question 2: Theoretical Yield of Benzoic acid ___________________ mol, ___________________ g
- Question 3: Actual Yield ___________________
- Question 4: Percentage Yield ___________________
- Question 5: Show your calculations.
- Question 6: Melting Point of Product _________________ (observed), _________________ (reported)