Lab 13 - Redox Reactions
Purpose
To determine relative oxidizing and reducing strengths of a series of metals and ions.Goals
-
1To explore the relative oxidizing and reducing strengths of different metals.
-
2To gain practice working with electrochemical cells.
-
3To use experimentally determined cell potentials to rank reduction half-reactions.
Introduction
The movement or transfer of electrons is central to our understanding of chemical reactions. The study of the transfer of electrons from one reactant to another is the study of electrochemistry. Electrons can move spontaneously from higher energy levels to lower energy levels within an atom. A similar movement can take place between two different chemical reactants. If there are electrons in one reactant that are at higher energy than unfilled orbitals of the other reactant, the high energy electrons can transfer to the unfilled orbitals at lower energy. This transfer of electrons from one chemical substance to another is known as an oxidation-reduction (redox) or electron transfer reaction. Consider the redox reaction (1) and Figure 1 below:( 1 )
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
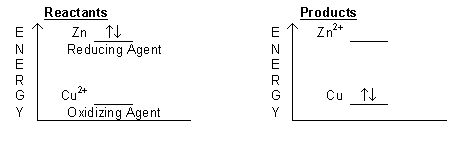
Figure 1: Energy Diagram for Reaction between Zinc Metal and Copper(II) Ion
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
are shown below. In this example, zinc loses two electrons and copper(II) accepts both.
( 2 )
Zn → Zn2+ + 2 e− (oxidation half-reaction, reducing agent)
( 3 )
Cu2+ + 2 e− → Cu (reduction half reaction, oxidizing agent)
( 4 )
Cu → Cu2+ + 2 e− (oxidation half-reaction)
( 5 )
Ag+ + 1 e− → Ag (reduction half-reaction)
( 6 )
2 Ag+ + Cu → 2 Ag + Cu2+ (net reaction)
Part A: Relative Reactivities
In Part A of this experiment, you will rank the relative strengths of oxidizing and reducing agents by observing if reactions occur or not. A visible change will accompany each reaction. A solid or gas will form, or a color change will occur. This indicates that the unfilled orbitals in the oxidizing agent are at lower energy than the filled orbitals of the reducing agent. The reaction is the result of electron transfer. If no such change is observed, no reaction has occurred. You will test three oxidizing agents, Cu2+, Mg2+ and MnO4-, to determine their relative reactivities. The solutions that will supply these ions are Cu(NO3)2, Mg(NO3)2 and KMnO4, respectively. The reduction half-reaction for each oxidizing agent is shown below in alphabetical order.( 7 )
Cu2+(aq) + 2 e−  Cu(s)
Cu(s)
 Cu(s)
Cu(s)( 8 )
Mg2+(aq) + 2 e−  Mg(s)
Mg(s)
 Mg(s)
Mg(s)( 9 )
MnO4−(aq) + 8 H+(aq) + 5 e−  Mn2+(aq) + 4 H2O(l)
Mn2+(aq) + 4 H2O(l)
 Mn2+(aq) + 4 H2O(l)
Mn2+(aq) + 4 H2O(l)( 10 )
Cu(s)  Cu2+(aq) + 2 e−
Cu2+(aq) + 2 e−
 Cu2+(aq) + 2 e−
Cu2+(aq) + 2 e−( 11 )
Mg(s)  Mg2+(aq) + 2 e−
Mg2+(aq) + 2 e−
 Mg2+(aq) + 2 e−
Mg2+(aq) + 2 e−( 12 )
Zn(s)  Zn2+(aq) + 2 e−
Zn2+(aq) + 2 e−
 Zn2+(aq) + 2 e−
Zn2+(aq) + 2 e−Part B: Half-Cell Potentials
When electrons are transferred spontaneously (downhill in free energy), they can do work in an external circuit if the half-reactions are separated into different compartments. This is how batteries work. Such devices are called galvanic cells. It is also possible to set up an electrolytic cell, in which an external voltage (energy source) is used to drive a redox reaction in the nonspontaneous direction. Many industrial processes involve electrolysis. An important example is the production of aluminum metal from its ore (Al2O3). Separating half-reactions also allows one to measure the energy difference between the electrons in the donor orbitals of a reducing agent and the acceptor orbitals of an oxidizing agent. You will combine a series of redox couples and measure the energy differences between them. This is typically performed in an electrochemical cell. One is shown in Figure 2 below.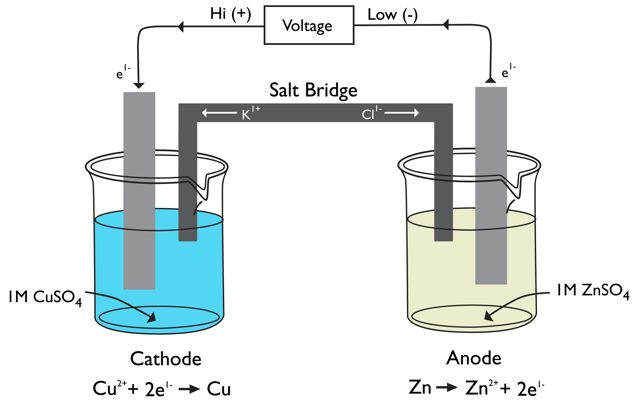
Figure 2: Electrochemical Cell for the Reaction between Copper Metal and Zinc Ion
( 13 )
Ecell = Ecathode − Eanode
Equipment
Part A: Relative Reactivities
-
1ceramic spot plate
-
330 mL beakers
-
1deionized water squirt bottle
Part B: Half-Cell Potentials
-
4electrochemical cell set-ups on the side shelf of the lab
Reagents
Part A: Relative Reactivities
- ~6 drops 0.1 M Cu(NO3)2
- ~6 drops 0.1 M Mg(NO3)2
- ~6 drops 0.1 M KMnO4 (acidic)
- ~9 drops 3% H2O2 solution
- ~9 drops 0.1 M KI
- ~9 drops phenolphthalein solution
- ~2 pieces Cu metal
- ~2 pieces Zn metal
- ~2 pieces Mg metal
- ~20 mL 3 M HCl
- ~30 mL tap water
Part B: Half-Cell Potentials
- 5 x 100 mL 0.1 M Cu(NO3)2 (in covered plastic container)
- 100 mL 0.1 M AgNO3 (in covered plastic container)
- 100 mL 0.1 M Pb(NO3)2 (in covered plastic container)
- 100 mL 0.1 M Zn(NO3)2 (in covered plastic container)
- 5 x ~3" copper wire
- ~3" silver wire
- ~3" lead wire
- ~3" zinc wire
Safety
The potassium permanganate solution (KMnO4) is a strong oxidizing agent; it is also acidic and corrosive. The solution of 3M HCl is acidic and corrosive. Both solutions can attack the skin and cause permanent damage to the eyes. If either of these solutions splashes into your eyes, use the eyewash immediately. Hold your eyes open and flush with water. If contact with skin or clothing occurs, flush the affected area with water. Have your lab partner notify your instructor about the spill. 3M HCl gives off acidic and irritating vapors. Add it carefully to your beakers in the fume hood on the side shelf. Avoid inhaling the vapors. The reducing agents produce hydrogen gas when exposed to water and/or acid. Keep the reactions away from ignition sources, and rinse acid off metal before discarding it. Do not tightly cap the waste container. As with all labs, be sure to wash your hands thoroughly after handling any chemicals and avoid touching your eyes and mouth during lab.Waste Disposal
The solutions from Part A1 of the experiment should be rinsed into the waste container for oxidizing agents. There will be a funnel in the container. Pour the contents of the well plate into the funnel, and then rinse the plate with water from a squeeze bottle. The metals from Part A2 of the experiment should be removed from the reactions with forceps, rinsed with water if they have been exposed to acid, blotted to remove excess water, and then discarded in the container for used metals. Do not tightly cap this container; hydrogen gas could build up pressure in it. All liquid waste should be put into the large waste container designated by your instructor. There is no waste for Part B of the experiment.Prior to Class
Please complete WebAssign prelab assignment. Check your WebAssign Account for due dates. Students who do not complete the WebAssign prelab are required to bring and hand in the prelab worksheet.Lab Procedure
Please print the worksheet for this lab. You will need this sheet to record your data. For this lab, Part A will be set up at your lab station. During the lab period, each pair should take turns going to the side shelf to record measurements for Part B.Part A1: Ranking Oxidizing Agents
-
1Obtain a ceramic well plate.
-
2Add 3 drops of Cu(NO3)2 solution to the first well, 3 drops of Mg(NO3)2 solution to the second well, and 3 drops of KMnO4 solution to the third well.
-
3Add 2 drops of H2O2 solution to each well. If something happened, write "R" (reaction) in Data Table A1 and make a brief note of what occurred in the space below it. If nothing happened, write "NR" (no reaction) in the space. Any oxidizing agent that reacted with the H2O2 is a stronger oxidizing agent than any which did not.
-
4If no reaction was observed, place three drops of the oxidizing agent in another well.
-
5Add three drops of KI solution to each well. If something happened, write "R" (reaction) in Data Table A1 and make a brief note of what occurred in the space below it. If nothing happened, write "NR" (no reaction) in the space. Any oxidizing agent that reacted with the KI is a stronger oxidizing agent than any which did not.
-
6Pour the contents of the well plate into the waste bottle for oxidizing agents and rinse it with your squirt bottle. The rinsings should also go into the waste bottle. Pour the contents of the well plate into the waste bottle for oxidizing agents and rinse it with your squirt bottle. The rinsings should also go into the waste bottle.
Table A: Reactions of Oxidizing Agents
Question 1: List the oxidizing agents in order, from weakest to strongest.
Question 2: Write half-reactions for the oxidizing agents in order, from weakest to strongest. (Hint: Remember that oxidizing agents get reduced.)
Part A2: Ranking Reducing Agents
-
1Obtain three 30 mL beakers and label them Cu, Mg, and Zn.
-
2Add 10 - 15 mL of tap water to each, then place a small piece of copper metal in the one labeled "Cu", magnesium metal to the one labeled "Mg" and zinc metal to the one labeled "Zn".
-
3To each beaker, add 3 drops of phenolphthalein indicator. If something happened, write "R" (reaction) in Data Table A2 and make a brief note of what occurred in the space below it. If nothing happened, write "NR" (no reaction) in the space. Any reducing agent that reacted with the water is a stronger reducing agent than any which did not.
-
4With the forceps provided by the waste jar, remove the metals from each of the three beakers. If no reaction occurred, rinse the metal with deionized water and place it on a paper towel to dry. This metal can be used in step 5. If a reaction did occur, place the metal in the Used Metal Jar. The liquids can be flushed down the sink with water.
-
5Rinse and dry the beakers, then place a new sample of the metals that did not react with water in the properly labeled beakers.
-
6Add about 10 mL of tap water to each beaker.
-
7Go to the side shelf fume hood and add 10 mL of 3 M HCl solution to each beaker. If something happened, write "R" (reaction) in Data Table A2 and make a brief note of what occurred in the space below it. If nothing happened, write "NR" (no reaction) in the space. Any reducing agent that reacted with the acid is a stronger reducing agent than any which did not.
-
8With the forceps provided near the waste jar, remove the metals from each of the beakers, rinse them with deionized water from a squeeze bottle and blot them dry. Place them in the Used Metal Jar. The liquids can be flushed down the sink with water.
-
9Wash and dry all your equipment and return it to the set-up area where you found it.
Table A2: Reactions of Reducing Agents
Question 3: List the reducing agents in order, from strongest to weakest.
Question 4: Write the half-reactions for the reducing agents in order, from weakest to strongest. (Hint: Remember that reducing agents get oxidized.)
Question 5: The strongest oxidizing agent is said to have the most positive potential and the strongest reducing agent has the most negative potential. Based on your observations, list all the half-reactions (as reductions) in order from most negative to most positive.
Question 6: Consider the reaction involving magnesium metal.
-
aWith what compound, element or ion did magnesium react?
-
bWrite a half-reaction for what happened to this chemical. You may use a Table of Standard Reduction Potentials for help.
-
cWrite the balanced equation for the reaction that occurred between magnesium metal and this chemical.
Question 7: You also observed a reaction with zinc metal.
-
aWith what compound, element or ion did zinc react?
-
bWrite a half-reaction for what happened to this chemical. You may use a Table of Standard Reduction Potentials for help.
-
cWrite the balanced equation for the reaction that occurred between zinc metal and this chemical.
Question 8: Based on your answers to Question 5, will either of these combinations produce a reaction?
-
aCu + Mg2+
-
bCu2+ + Mg
Part B: Half-Cell Potentials
-
1Go to the side shelf where the electrochemical cells are set up for Part B. If the salt bridges are not inserted into the solutions as shown in Figure 3 below, please let your instructor know.

Figure 3: Electrochemical Cell Setup
-
2For the Copper-Copper cell, attach the black alligator clip lead to one of the copper wires in copper solution. The copper wire should not be completely submerged.
-
3Next attach the red alligator clip lead to the other copper wire in copper solution. This wire should not be completely submerged either.
-
4Measure the potential (in millivolts) and record it in Data Table B1. This value should be very close to 0.0 mV since there is no potential difference between copper and itself. If you do not find this result, consult your instructor.
-
5When you are finished taking your measurement, remove the leads from the wires and leave the set up the way you found it.
-
6Repeat steps 2 - 5 for the three remaining cells, always attaching the black alligator clip lead to the copper half-cell. The Silver-Copper cell should have a positive cell potential. If it does not, consult your instructor.
-
7Convert the cell potentials in Data Table B1 to volts.
-
8Enter the four couples into Data Table B2, arranging them in order from most negative potential to most positive potential.
-
9We have treated the Cu2+/Cu couple as a reference point for our measurements. However, the standard hydrogen electrode (SHE) is defined by international convention as the zero volt reference. The reduction potential of Cu2+/Cu is +0.34 V relative to this standard. Therefore by adding +0.34 V to each of the potentials you measured vs Cu2+/Cu will convert them to potentials vs SHE. Enter these values in Data Table B2, column 3.
-
10Refer to the table of Standard Reduction Potentials given on the inside front cover of this lab manual to find the actual standard reduction potentials of these four couples. Enter these values in Data Table B2, column 4.
Table B1: Cell Potentials vs a Cu2+/Cu Couple
Table B2: Cell Potentials in Order, with Half-Reactions
Question 9: Based on the order obtained by experiment,
-
aWhich species has the highest energy filled or partially filled orbitals?
-
bWhich species has the lowest energy unfilled or partially filled orbitals?
-
cWhich species is the strongest reducing agent?
-
dWhich species is the strongest oxidizing agent?
Question 10: Using the order you found in Data Table B2 for the cell potentials, write the half-reaction for each half-cell. Write the reactions as reductions.
Question 11: The Mg2+/Mg couple was not tested when measuring half-cell potentials. Based on its behavior in Part A, where would you place it in Data Table B2? (If you are doing Part B first, return to this question after completing both parts of the lab.)
-
11Before leaving, go to a computer in the laboratory and enter your results in the In-Lab assignment. If all results are scored as correct, log out. If not all results are correct, try to find the error or consult with your lab instructor. When all results are correct, note them and log out of WebAssign. The In-Lab assignment must be completed by the end of the lab period. If additional time is required, please consult with your lab instructor.