Lab 7 - Buffers
Purpose
To prepare buffers and measure the pH of each, and to prepare a buffer at a specific pH.Goals
-
•To learn to prepare buffers by both the direct and indirect methods.
-
•To learn to identify solutions that are buffers.
-
•To understand how a buffer resists changes in pH upon addition of acid or base solutions.
Introduction
In dilute aqueous solutions, weak acids are slightly dissociated. They produce a small concentration of hydronium ion (H3O+) and an equal concentration of the conjugate base of the acid. Such dissociation reactions are equilibria, and equilibrium mathematics can be used to calculate concentrations of the species present in solution. Consider formic acid (CH2O2); its dissociation constant (Ka) is 1.7 × 10–4. Incidentally, formic acid is what red ants inject when they bite. The concentration of H3O+ present in a 0.010 M solution of formic acid can be calculated from the equilibrium expression and a reaction table.( 1 )
| HCOOH(aq) | + | H2O(l) | 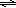 | H3O+(aq) | + | HCOO−(aq) | |
| initial | 0.010 | 0 | 0 | ||||
| Δ | −x | +x | +x | ||||
| equilibrium | 0.010 − x | x | x | ||||
( 2 )
Ka = 1.7 × 10−4 =
=
| [H3O+ ][HCOO− ] |
| [HCOOH] |
| x2 |
| 0.010 − x |
HCOO−.
For this calculation, the quadratic formula was used. If one makes the simplifying assumption that x is small relative to [HCOOH], the calculated value is 0.0013 M.† Expressing [H3O+] as pH,
( 3 )
pH = −log[H3O+ ] = −log(0.0012) = 2.92.
( 4 )
HCOONa(s) + H2O(l) → Na+(aq) + HCOO−(aq)
| HCOOH(aq) | + | H2O(l) | 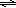 | H3O+(aq) | + | HCOO−(aq) | |
| initial | 0.010 | 0 | 0 | ||||
| Δ | −x | +x | +x | ||||
| equilibrium | 0.010 − x | x | x | ||||
| HCOOH(aq) | + | H2O(l) | 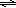 | H3O+(aq) | + | HCOO−(aq) | |
| initial | 0.010 | 0 | 0 | ||||
| Δ | −x | +x | +x | ||||
| equilibrium | 0.010 − x | x | x | ||||
| HCOOH(aq) | + | H2O(l) | 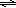 | H3O+(aq) | + | HCOO−(aq) | |
| initial | 0.010 | 0 | 0 | ||||
| Δ | −x | +x | +x | ||||
| equilibrium | 0.010 − x | x | x | ||||
( 5 )
pH = pKa + log
or pH = pKa + log
.
 |
| [base] |
| [acid] |
 |
 |
| moles of base |
| moles of acid |
 |
( 6 )
HA + H2O 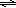 H3O+ + A−.
H3O+ + A−.
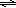 H3O+ + A−.
H3O+ + A−.( 7 )
Ka =
.
| [H3O+ ][A− ] |
| [HA] |
( 8 )
[H3O+] =
.
| Ka × [HA] |
| [A− ] |
( 9 )
pH = −log[H3O+ ] = −log Ka − log
.
 |
| [HA] |
| [A−] |
 |
pKa = −log Ka and −log([HA]/[A− ]) = log([A− ]/[HA]).
Substituting these terms into equation 9pH = −log[H3O+ ] = −log Ka − log
.
:
 |
| [HA] |
| [A−] |
 |
( 10 )
pH = pKa + log
.
 |
| [A−] |
| [HA] |
 |
pH = pKa + log
or pH = pKa + log
.
shows that pH can be found using either concentrations of acid and base, or the number of moles of each. This follows from the fact that the volume term is the same for the acid and its conjugate base, and cancels in the calculation.
The Henderson-Hasselbalch equation shows that the pH of a buffer is close to the pKa of the weak acid from which it is made. The exact pH is dependent on the ratio  |
| [base] |
| [acid] |
 |
 |
| moles of base |
| moles of acid |
 |
[A− ]/[HA]. If [HA] = [A− ],
log([A− ]/[HA]) = 0
and the pH of the buffer will be exactly the pKa of the acid. If one wishes to make a buffer of a specific pH, one selects an acid with a pKa near that value and adjusts the ratio of [A− ]/[HA]
to obtain the desired buffer. Suppose we want a pH 4.00 formate buffer. The pKa of formic acid is −log(1.7 × 10−4) = 3.77.
( 11 )
| pH | = | pKa + log
| ||||
| 4.00 | = | 3.77 + log
| ||||
| = | 1.70 |
H2PO4−/HPO42−
conjugate pair has a pKa of about 7.2, so it should be a good system to use for buffers in the pH range of about 6.5 to 8.0. The HPO42−/PO43−
conjugate pair has a pKa of about 12.3, so it should be a good system to use for buffers in the pH range of about 11.5 to 13.0.
Equipment
-
2 30 mL beakers
-
2 100 mL volumetric flasks
-
1 10.0 mL volumetric pipet
-
1 pipet bulb
-
1 50 mL graduated cylinder
-
1 10 mL graduated cylinder
-
1 50 mL beaker
-
1 100 mL beaker
-
1 glass stir rod
-
1 MicroLab interface
-
1 MicroLab pH measurement instruction sheet
-
1 pH electrode in pH 7.00 buffer
-
1 ring stand
-
1 clamp
-
1 250 mL beaker for electrode rinsings
-
1 deionized water squirt bottle
-
1 box Kimwipes
Reagents
-
~10 mL 6.0 M acetic acid (HC2H3O2)
-
<10 g solid sodium acetate trihydrate (NaC2H3O2 · 3 H2O)
-
~25 mL 1.0 M NaOH
-
3 g solid Na2HPO4 · 7 H2O
-
TBD solid NaH2PO4 · H2O or solid Na3PO4 · 12 H2O
-
~15 mL pH 4.00 buffer
-
~15 mL pH 7.00 buffer
-
~15 mL pH 10.00 buffer
-
1.0 M NaOH in a dropper bottle for Part C
-
1.0 M HCl in a dropper bottle for Part C
Safety
Acetic acid, HCl and NaOH are corrosive. They can attack the skin and cause permanent damage to the eyes. If one of these solutions splashes into your eyes, use the eyewash immediately. Hold your eyes open and flush with water. If contact with skin or clothing occurs, flush the affected area with water. Have your lab partner notify your instructor about the spill.Waste Disposal
All solutions can be flushed down the sink with water.Prior to Class
Please read the following sections of Lab Safety and Practices: Please read the following sections of Lab Equipment: Please review the following videos:Lab Procedure
Please print the worksheet for this lab. You will need this sheet to record your data. In this experiment, you will be using pH electrodes connected to the MicroLab interface. pH electrodes have a thin glass bulb at the tip. They break easily and are costly to replace. Be careful not to shove the electrode into the bottom of a beaker or drop the electrode. There is a protective guard around the tip, which should remain in place at all times. The guard will not protect against careless treatment. Please use extreme care when using this equipment. Best results in using the electrodes are obtained if:-
•Electrodes are kept in standard pH 7 buffer solution when not in use.
-
•Immediately prior to use, the electrodes are rinsed with deionized water and gently blotted with a tissue, then placed in the test solution.
-
•The electrodes are rinsed and blotted again after the measurement and returned to the pH 7 buffer solution.
Part A: Acetate Buffer by the Direct Method
1
Open the MicroLab program.
2
Make sure the pH electrode is connected to the interface.
3
Calibrate the pH electrode using the MicroLab instructions provided in the lab. The calibration standards for the pH electrode will be a pH = 4.00 (red) buffer solution, a pH = 7.00 (yellow) buffer solution, and a pH = 10.00 (blue) buffer solution. Use about 15 mL of each in 30 mL beakers.
4
After the calibration is complete, configure the MicroLab program to collect data as described in the instructions provided in the lab.
5
Using the 10.0 mL volumetric pipet and a 100.0 mL volumetric flask, prepare 100.0 mL of 0.60 M HC2H3O2 by diluting the 6.0 M stock solution provided. (Hint: Remember your prelab exercise!) Be sure to condition your pipet before using it.
6
Using the other 100.0 mL volumetric flask, prepare 100.0 mL of 0.60 M sodium acetate solution by dissolving solid sodium acetate trihydrate (NaC2H3O2 · 3 H2O) in water and diluting to a total volume of 100.0 mL. (Hint: Remember your prelab exercise!)
7
Use a graduated cylinder to measure 30 mL of the 0.60 M acetic acid solution you prepared into a 50 mL beaker. Measure the pH of this solution and record it in Table A as solution 1A. Make sure the electrode bulb is fully immersed before measuring.
8
Add 10 mL of your sodium acetate solution to the beaker containing acetic acid and stir with a clean stirring rod. Measure the pH of the solution and record it in Table A as solution 2A. After the measurement is complete, the solution may be discarded.
9
Use a graduated cylinder to measure 30 mL of the 0.60 M sodium acetate solution you prepared into a 50 mL beaker. Measure the pH of the solution and record it in Table A as solution 3A.
10
Add 10 mL of your acetic acid solution to the beaker containing sodium acetate and stir with a clean stirring rod. Measure the pH of the solution and record it in Table A as solution 4A. After the measurement is complete, the solution may be discarded.
11
Make solution 5A by mixing 20 mL of acetic acid solution with 20 mL of sodium acetate, stirring well. Measure its pH and record it in Table A as solution 5A.
Part B: Acetate Buffer by the Indirect Method
1
Place 30 mL of your 0.60 M acetic acid in a clean 100 mL beaker. Measure the pH of the solution and record it in Table B as solution 1B.
2
Determine whether or not this solution is a buffer solution, and enter your decision in Table B.
3
Add 4 mL of 1.0 M NaOH and mix the solution thoroughly. Measure the pH of the solution and record it in Table B as solution 2B.
4
Add in succession another 5 mL, then 6 mL and finally 10 mL of 1.0 M NaOH, mixing thoroughly and recording the pH after each addition.
5
Enter the cumulative number of moles of NaOH added in each step in Table B. After the last addition, a total of 25 mL of NaOH should have been added.
6
Decide whether or not each solution is a buffer and enter your decision in Table B.
Part C: Phosphate Buffer by the Direct Method
1
Your lab instructor will assign you a target pH; write it in Table C. You need to generate 100 mL of the buffer solution starting with 3.00 g of Na2HPO4 · 7 H2O.
2
Weigh out 3 g of Na2HPO4 · 7 H2O and record the exact mass in Table C. Transfer it carefully to a 100 mL volumetric flask. Add about 60 mL of deionized water, stopper the flask, and shake it to dissolve the solid. This will take a while.
3
Weigh out the calculated amount of the second phosphate compound and carefully add it to your volumetric flask. Stopper the flask and shake until all solid is dissolved. Then add deionized water up to the mark of your flask.
4
Pour some of the buffer solution into a beaker and measure its pH. Record the result in Table C.
5
If the pH is different than the target pH, adjust it by adding 1.0 M HCl or 1.0 M NaOH dropwise until the desired pH is achieved. In your notebook, describe the action taken to adjust the pH of the buffer, e.g. "added 3 drops of 1.0 M HCl."
6
After your last measurement, stop and close the MicroLab software. Rinse all of your glassware with water, dry it and return it to the set-up area where you found it. Make sure the pH electrode is submerged in the pH 7 buffer solution.
7
Before leaving, enter your results in the in-lab assignment. If all results are scored as correct, log out. If not all results are correct, try to find the error or consult with your lab instructor. When all results are correct, note them and log out of WebAssign. The in-lab assignment must be completed by the end of the lab period. If additional time is required, please consult with your lab instructor.