Lab 5 - Determination of an Equilibrium Constant
Purpose
To determine the equilibrium constant for the reaction:Fe3+ + SCN–  FeSCN2+
FeSCN2+
 FeSCN2+
FeSCN2+Goals
-
•To gain more practice using a pipet properly.
-
•To gain more practice diluting stock solutions.
-
•To gain more practice using a spectrophotometer.
-
•To gain practice plotting a calibration curve and use it to determine the concentration of an unknown solution.
Introduction
A typical chemical equation has the following form:( 1 )
aA + bB → cC + dD.
( 2 )
aA + bB  cC + dD
cC + dD
 cC + dD
cC + dD( 3 )
Keq =
.
| [C]c[D]d |
| [A]a[B]b |
Keq =
.
. The value of Keq varies with temperature; therefore, the temperature at which the equilibrium constant was determined must be referenced.
In this laboratory experiment, a combination of solution chemistry, stoichiometry and spectrophotometric analysis will be used to determine the equilibrium constant for a reaction between iron (III) ion (Fe3+) and thiocyanate ion (SCN–). In acidic solution, these ions form a blood-red complex ion as shown in equation 4.
| [C]c[D]d |
| [A]a[B]b |
( 4 )
Fe3+(aq) + SCN–(aq)  FeSCN2+(aq)
FeSCN2+(aq)
 FeSCN2+(aq)
FeSCN2+(aq)Fe3+(aq) + SCN–(aq)  FeSCN2+(aq)
FeSCN2+(aq)
 FeSCN2+(aq)
FeSCN2+(aq)( 5 )
K =
| [FeSCN2+] |
| [Fe3+][SCN−] |
Fe3+
and 2.0 M SCN−.
The term "initial concentration" can be confusing. Even though the reaction appears to go instantaneously upon mixing the reactants, the "initial concentrations" in the reaction table are those after dilution has been taken into consideration but before any reaction occurs. Thus, the initial line in the reaction table for mixing equal volumes of 2.0 M Fe3+
and 2.0 M SCN−
should have entries of 1.0 M under Fe3+
and SCN−
due to dilution. The initial concentration of FeSCN2+
is 0.0 M. In our example, you might measure an equilibrium (final) concentration of 0.6 M FeSCN2+.
With the final concentration of the product, you can determine the change in product concentration and, therefore, the changes in the reactant concentrations. The reaction table is shown below.
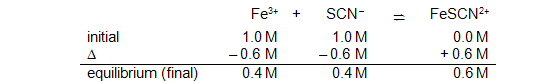 In this experiment, 0.2 M HNO3 serves as the solvent. The acid adds a large (compared to the reactants) amount of H+. This prevents side reactions such as the formation of FeOH2+, a brownish species that can affect the results. The acid concentration is high enough that it is not affected by the reaction and remains constant at 0.2 M.
You will prepare six standard solutions of
In this experiment, 0.2 M HNO3 serves as the solvent. The acid adds a large (compared to the reactants) amount of H+. This prevents side reactions such as the formation of FeOH2+, a brownish species that can affect the results. The acid concentration is high enough that it is not affected by the reaction and remains constant at 0.2 M.
You will prepare six standard solutions of FeSCN2+
to calibrate a spectrophotometer. A fair question is "How do I know the concentration of FeSCN2+ in my standard solutions if it is in equilibrium with Fe3+ and SCN–?" In the standard solutions, the concentration of Fe3+ is much higher than that of SCN–. This forces the equilibrium as far to the right (toward FeSCN2+) as possible. Therefore, the concentration of FeSCN2+ in a standard solution will be very nearly equal to the initial concentration of SCN– used in preparing it. The absorbance measurement at 470 nm will correlate to the concentration of complex ion, and an accurate calibration curve (Beer's Law plot) can be obtained. Recall that the calibration curve gives you a relationship between the concentration of a species in solution and its absorbance at a given wavelength: (A = εlc). Using the linear regression of the calibration curve in Part A, you will determine the concentration of FeSCN2+ ion in each of five equilibrium mixtures in Part B. An equilibrium constant can then be determined for each mixture; the average should be the equilibrium constant value for the formation of the FeSCN2+ ion.
In Part A of this experiment, you will prepare FeSCN2+ solutions of known concentrations, measure their absorbances at 470 nm, and produce a calibration curve. In Part B, you will make equilibrium mixtures of Fe3+, SCN–, and FeSCN2+. You will determine the concentration of FeSCN2+ from its absorbance at 470 nm and your calibration curve from Part A. Then using reaction tables, you will calculate the equilibrium concentrations of Fe3+ and SCN–, and determine the equilibrium constant for the formation of FeSCN2+.
Equipment
-
1 MicroLab spectrophotometer
-
1 MicroLab spectrophotometer instruction sheet
-
6 vials
-
3 serological pipets
-
1 pipet bulb
-
3 30 mL beakers for reagents
-
6 13 × 100 mm test tubes for mixtures
-
6 stoppers
-
1 test tube rack
-
1 250 mL beaker for waste
-
1 deionized water squirt bottle
Reagents
-
~10 mL 0.100 M Fe(NO3)3 in 0.2 M HNO3
-
~10 mL 5.00 × 10–4 M NaSCN in 0.2 M HNO3
-
~15 mL 0.002 M Fe(NO3)3 in 0.2 M HNO3
-
~15 mL 0.002 M NaSCN in 0.2 M HNO3
Safety
Nitric acid is listed as a corrosive. Corrosives can attack the skin and cause permanent damage to the eyes. Nitric acid and iron(III) nitrate are listed as oxidants. Sodium thiocyanate is listed as toxic and an irritant. With the exception of nitric acid, the concentrations of all these materials are quite low, however. If you spill any of these chemicals on skin or clothing, flush the area immediately with water.Waste Disposal
All of the solutions prepared in this experiment, as well as excess NaSCN solution, should be discarded in the waste container. You may wish to have a waste beaker in your work area to collect waste while you are doing the experiment. Make sure it is labeled. Always remember not to overfill the waste bottle. If your waste bottle is full, please alert your lab instructor.Prior to Class
Please read the following section of Lab Safety and Practices: Please read the following section of Lab Equipment: Please review the following videos:Lab Procedure
Please print the worksheet for this lab. You will need this sheet to record your data.Part A: Preparation of Standard Solutions and Beer's Law Plot
1
A spectrophotometer will be set up in your work area. Make sure it is turned on and allow it to warm up.
2
While the spectrophotometer is warming up, obtain three serological pipets, and label a beaker for waste.
3
Obtain about 10 mL of 0.100 M Fe(NO3)3
in 0.2 M HNO3
in a small, clean, dry beaker. Obtain about 10 mL of 5.00 × 10−4
M NaSCN in 0.2 M HNO3
in another beaker. Label the solutions so you do not mix them up.
4
Condition one pipet for each of the solutions you obtained in step 3 and one for deionized water. This procedure is shown in the Pipeting Techniques video under Instrumentation and is described in the Volumetric Glassware section of the Introductory Material of this lab manual.
5
Using the conditioned pipets, add the amounts of the Fe3+
solution, SCN−
solution and water listed in Table A for the Blank and Solutions 1A - 5A to six labeled test tubes. Stopper each test tube and invert a few times to mix each solution.
6
Once the spectrophotometer is warmed up, take a spectrum with your Blank solution. To condition your vial, carefully pour a small amount of the Blank solution into a vial and pour it out to waste. Refer to the MicroLab spectrophotometer instructions provided in lab.
7
Condition a vial using Solution 1A, refill the vial, measure its absorbance at 470 nm and record this value in Table A. When finished, retain this sample in your vial until you have completed your calibration plot. Students often choose to label a sheet of paper with positions 1A, 2A, etc., placing each vial on the appropriate position.
8
Repeat step 7 for each of the remaining solutions from Table A.
9
The MicroLab software will plot the absorbance of the FeSCN2+
solutions as a function of their concentrations. The trendline and R2
value are displayed. If your plot is linear with an R2
value of 0.9 or greater, continue the experiment. If your R2
value is low, consult with your TA. Record the trendline and R2
value in Table A. Do not close the MicroLab file, as this calibration will be used to determine concentrations in Part B.
10
Safely dispose of the calibration solutions in your labeled waste beaker.
11
Discard any remaining 0.100 M Fe(NO3)3
in 0.2 M HNO3
and 5.00 × 10−4
M NaSCN in 0.2 M HNO3
in the labeled waste container. Rinse and dry your beakers for use in Part B.
Part B: Preparation of the Equilibrium Mixtures and Absorbance Measurements
1
Obtain about 15 mL of 0.002 M Fe(NO3)3
in 0.2 M HNO3
in a small, clean, dry beaker. Obtain about 15 mL of 0.002 M NaSCN in 0.2 M HNO3
in another beaker. Label the solutions so you do not mix them up.
2
Re-condition your pipets with the new solutions of Fe3+
and SCN−.
3
Using the conditioned pipets, add the amounts of the Fe3+
solution, SCN−
solution and water required for Solutions 1B - 5B listed in Table B to five labeled test tubes. Stopper each test tube and invert a few times to mix the solutions.
4
Measure the absorbance of each solution as in Part A and record them in Table B.
5
When you are finished taking measurements, collect all your waste and place it in the waste bottle in the lab, making sure not to overfill it. Rinse and dry all your glassware with water and return it to the set-up area where you found it. Close the MicroLab software.
6
Remember to show your TA your calibration curve, reaction table, and equilibrium constant calculation. Your TA will manually grade the results and enter your score into WebAssign.
7
Before leaving, enter your results in the in-lab assignment. If all results are scored as correct, log out. If not all results are correct, try to find the error or consult with your lab instructor. When all results are correct, note them and log out of WebAssign. The in-lab assignment must be completed by the end of the lab period. If additional time is required, please consult with your lab instructor.