Experiment 8 - Amide Preparation
Objective
In this experiment, the amide, 2-(N-acetylamino)benzoic acid (N-Acetylanthranilic acid) will be prepared by the reaction of 2-aminobenzoic acid (anthranilic acid) with acetic anhydride.Introduction
Amides are another one of the many functional groups encountered in the study of Organic Chemistry. The amide is identified by having a carbonyl unit (C=O) bound to a Nitrogen unit (NR2, R can be H).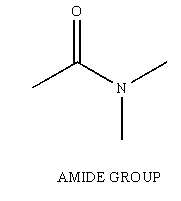
Figure 1
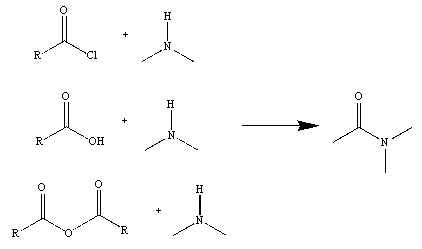
Figure 2

Figure 3
Pre-Lab
Complete the pre-lab assignment in WebAssign.Procedure
Note: Make sure that the reaction is performed under the bench hoods (i.e., not out in the middle of the bench top). Acetic anhydride is a lachrymator (look up what this means).
1
Place 1 g of anthranilic acid and 3-4 mL of acetic anhydride in a 25 mL Erlenmeyer flask.
2
Warm the mixture to boiling (gently) on a hot plate set to 260°C. All solid should dissolve.
3
Heat for a period of 15 minutes.
4
Allow to cool to room temperature.
5
Add 2 mL of water to the mixture.
6
Heat the mixture to boiling again.
7
Slowly let the reaction mixture cool to room temperature (i.e., no ice bath or cold tap water) so that the solid formed will have larger crystals.
8
Isolate the product by vacuum filtration (here is a video that shows you how to do a vacuum filtration).
9
Wash with a small amount of cold water while still on the Buchner funnel.
10
Determine the yield, percentage yield, and melting point of the product. Here is a video that shows you how to take a melting point.
Waste Disposal
1
The filtrate should be placed in a beaker designated by your TA to be neutralized.
2
Any solid product should be added to the "SOLID WASTE" container.
3
Melting point capillaries and test tubes should be thrown in broken glass.
In-Lab Questions
Download and print the following worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of anthranilic acid used ___________________ g, ___________________ mol
- Question 2: Theoretical Yield of product _____________________
- Question 3: Actual Yield of product _____________________
- Question 4: Percentage Yield _____________________
- Question 5: Melting Point ___________________ (observed), ___________________ (actual)
- Question 6: Record your calculations.