The following examples illustrate entry of some common notation.
|
Subject |
Scenario |
Type this |
To display this |
|---|---|---|---|
|
Molecules |
Using subscripts to format molecular ratios in chemical formulas |
|
H2O |
|
Simple Ions |
Entering charges |
|
Ca2+ |
|
Molecular or Compound Ions |
Entering charges and molecular ratios |
|
SO42- |
|
Complex Ions |
Grouping with subscripts and superscripts |
|
[Co(SCN)2(H2O)4]+ |
|
Isotope |
Entering an isotopic mass number in the so-called M/A or M/Z format |
|
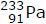
|
|
Chemical Reactions |
Entering a combination of correctly formatted chemical formulas and symbols |
|
2 H2O2 → 2 H2O + O2 |
|
Chemical Reactions with States of Matter |
Entering a combination of correctly formatted chemical formulas with their respective states of matter and symbols |
|
CH4(g) + 4 S(s) → CS2(l) + 2 H2S(g) |
|
Electron Configuration |
Using complete notation |
|
1s2 2s2 2p5 |
|
Electron Configuration |
Using noble gas notation |
|
[He] 2s2 2p5 |
|
Equilibrium Expressions |
Including a stacked fraction and multiplication dots |
|
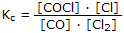
|
|
Electrochemical Cell Notation |
Enter cell line notation. |
|
Mg(s) | Mg2+(aq) || Zn2+(aq) | Zn(s) |