[Derivations are an essential part of a complete physics education. These multi-part questions test student instincts and understanding of the derivation process by stepping them through the process.]
Complete the derivation of
by substituting the wave function
ψ(x, y, z) = A sin(k1x) sin(k2y) sin(k3z)
into
Taking the derivatives we find (Use the following as necessary: k1, k2, k3, and ψ.)
∇2ψ =

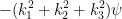
so the Schrödinger equation becomes (Use the following as necessary: k1, k2, k3, ℏ, m and ψ.)
| − ∇2ψ | = |

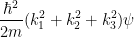
|
| = | Eψ |
The quantum numbers ni are related to ki by (Use the following as necessary: ni and Li.)
ki =

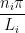
so
What is the origin of the three quantum
numbers?
Solution or Explanation
Taking the derivatives we find
∇2ψ = −(k12 + k22 + k32)ψ
so the
Schrödinger equation becomes
− ∇2ψ =
(
k12 +
k22 +
k32)
ψ =
Eψ.
From the boundary conditions
so
The three quantum numbers come directly from the three boundary conditions.