Acceptable values for the principle quantum number
n are
n = 1, 2, 3, 4,
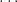
For each value of n, there are n possible values for the orbital angular momentum quantum number ℓ and they are
-
ℓ = 0, 1, 2, 3, ..., n − 1
-
ℓ = 0
is associated with s
-
ℓ = 1
is associated with p
-
ℓ = 2
is associated with d
-
ℓ = 3
is associated with f
and so forth.
For each value of
ℓ, we may have
2ℓ + 1
values of
mℓ
and they range from
−ℓ
to
+ℓ
in steps of 1 with values of
−ℓ
,
−ℓ + 1
,
−ℓ + 2,
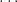
, 0, +1, +2,
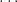
, +
ℓ − 1
,
+ℓ.
For each value of
mℓ
there are two acceptable values of
ms
and they are
ms = ±1/2.
Applying the above to atoms of hydrogen through boron we have the following.
Hydrogen (with 1 electron) which has quantum numbers
(n, ℓ, mℓ, ms)
equal to
(1, 0, 0, ±1/2)
when in its lowest state. Note that in the absence of a magnetic field, the
ms = +1/2
state is degenerate with the
ms = −1/2
state. As a result, we may write
Helium (with 2 electrons) which have quantum numbers
(1, 0, 0, +1/2)
and
(1, 0, 0, −1/2)
. Note that the
n = 1 shell is now full (all possible quantum numbers associated with
n = 1 have been used.) As a result, we may write
Lithium (with 3 electrons). Two of the electrons will fill the
n = 1 shell and the third
will have quantum numbers
(2, 0, 0, ±1/2)
. Note that in the absence of a magnetic field, the
ms = +1/2
state is degenerate with the
ms = −1/2
state. As a result, we may write
Beryllium (with 4 electrons). Two electrons fill the
n = 1 shell and the other two have quantum numbers
(2, 0, 0, +1/2)
and
(2, 0, 0, −1/2)
. Note that the
n = 2,
ℓ = 0
(or
s) subshell is now full (all possible quantum numbers associated with
n = 2 and ℓ = 0
have been used). As a result, we may write
Boron: (with 5 electrons). Two electrons fill the
n = 1 shell, two fill the
n = 2, ℓ = 0
subshell and the remaining electron goes into the
n = 2, ℓ = 1
subshell. As a result, we may write