
See adjacent tab for a text description of the label.
The label is split into three sections.
The first section reads:
- RANBAXY
- NDC 63304-954-01
- CEFACLOR For Oral Suspension USP
- 125 mg/5 mL
- 75 mL (when mixed)
- SHAKE WELL BEFORE USE
- Rx only
- Manufactured for: Ranbaxy Pharmaceuticals Inc. Jacksonville, FL 32216 USA
- by: Ranbaxy Laboratories Ltd. New Delhi - 110 019, India
- Usual Dosage: Children, 20 mg/kg a day (40 mg/kg in otitis media) in three divided doses. Adults, 250 mg three times a day, See literature for complete dosage information.
- Contains Cefaclor monohydrate equivalent to 1.875 g cefaclor in a dry, pleasantly flavored mixture.
- Prior to Mixing, store at 20-25°C (68-77°F). (See USP Controlled Room Temperature). Protect from moisture.
- Directions for Mixing: Add 53 mL of water in two portions to dry mixture in the bottle. Shake well after each addition. Each 5 mL (Approx. one teaspoonful) will then contain Cefaclor USP monohydrate equivalent to 125 mg anhydrous cefaclor. Over size bottle provides extra space for shaking.
- Store in a refrigerator. May be kept for 14 days without significant loss of potency. Keep tightly closed. Discard unused portion in 14 days.
- LOT:
- EXP:

See adjacent tab for a text description of the label.
The label is split into three sections. The first section reads:
- 50 Tablets
- Rx only
- NDC 0025-1821-50
- Flagyl® (metronidazole USP)
- 500 mg
- Pharmacist: Dispense in a well-closed, light-resistant, child-resistant container.
- SEARLE
- Store below 77°F (25°C).
- Note: This package is not child-resistant. Keep out of the reach of children.
- Usual Adult Dosage: See attached materials.
- G.D. Searle & Co. Chicago IL 60680 USA
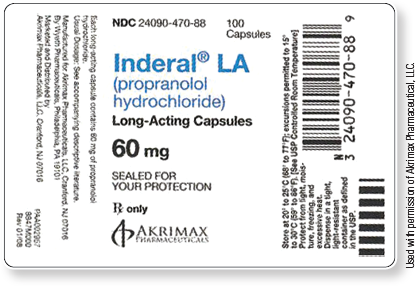
See adjacent tab for a text description of the label.
The label is split into three sections.
The first section reads:
- NDC 24090-470-88
- 100 Capsules
- Inderal® LA (propranolol hydrochlroide)
- Long-Acting Capsules
- 60 mg
- SEALED FOR YOUR PROTECTION
- Rx only
- AKRIMAX PHARMACEUTICALS
- Each long-acting capsule contains 60 mg of propranolol hydrochloride.
- Usual Dosage: See accompanying descriptive literature.
- Manufactured for: Akrimax Pharmaceuticals, LLC, Cranford, NJ 07016
- By Wyeth Pharmaceuticals, Philadelphia, PA 19101
- Marketed and Distributed by Akrimax Pharmaceuticals, LLC, Cranford, NJ 07016
- Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]
- Protect from light, moisture, freezing, and excessive heat.
- Dispense in a tight, light-resistant container as defined in the USP.

See adjacent tab for a text description of the label.
The label is split into three sections.
The first section reads:
- NDC 0069-3230-66
- 100 Capsules
- Feldene® (piroxicam)
- 20 mg
- Pfizer
- Pfizer Labs Division of Pfizer Inc, NY, NY 10017
- Store below 86°F (30°C)
- Dispense in tight, light-resistant containers (USP).
- DOSAGE AND USE See accompanying prescribing information. One capsule per day.
- Each capsule contains 20 mg piroxicam.
- IMPORTANT: This closure is not child-resistant.
- CAUTION: Federal law prohibits dispensing without prescription.
- 6505-01-137-4628
- 05-4300-00-5
- MADE IN USA
- 1292
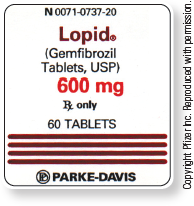
See adjacent tab for a text description of the label.
The label reads:
- N 0071-0737-20
- Lopid® (Gemfibrozil Tablets, USP)
- 600 mg
- Rx only
- 60 TABLETS
- PARKE-DAVIS