Measurements of a Table
Back to TopObjectives
-
•to practice the concepts of significant figures, mean value, standard deviation of the mean, and normal distribution by making multiple measurements of the length and width of a table
-
•to estimate and measure the area of the laboratory table and report it with the correct number of significant figures and in the correct format
-
•to practice the propagation of errors by determining the uncertainty in the measurement of the area of the table
-
•to understand the difference between precision and accuracy
Equipment
20-cm wooden rod, laboratory table, measuring tape or 2-m metric ruler, Graphical Analysis (GA) or Logger Pro Software
Figure 1
Introduction and Theory
Physics is largely a science of measurements. Experience has shown that no measurement is exact. All measurements have some degree of uncertainty due to the limitations of instruments and the people using them. In science, it is critically important to specify that uncertainty and include its reliable estimate in the final statement of the result of the measurement. Two concepts associated with measurements are accuracy and precision. The accuracy of the measurement refers to how close the measured value is to the true or accepted value. For example, if you used a balance to find the mass of a known, standard 100.00-g mass, and you got a reading of 78.55 g, your measurement would not be very accurate. In general, the experimantal results are considered accurate if the percent discprepancy/difference is within 10–15%, depending on the experimental setup used to prove the theoretical concept. Precision is the degree of exactness or refinement of measurements. Precision has nothing to do with the true or accepted value of a measurement, so it is quite possible to be very precise and totally inaccurate. In many cases, when precision is high and accuracy is low, the fault can lie with the instrument. If a balance or a thermometer is not working correctly, they might consistently give a systematic error (inaccurate answers) resulting in high precision and low accuracy. In general, the experimental results are considered to be precise if the relative uncertainty is less than 5–10%, depending on the experimental setup used to prove the theoretical concept. One important distinction between accuracy and precision is that accuracy can be determined by only one measurement, while precision can only be determined with multiple measurements. A measurement can be accurate but not precise, precise but not accurate, neither, or both. For example, if an experiment contains a systematic error, then increasing the sample size generally increases precision, but it does not improve accuracy. Eliminating the systematic error improves accuracy, but it does not change precision. Commonly, the best estimate of the true value of the measured quantity x can be obtained by calculating the mean (average) value of some significant N number of measurements of that quantity repeated using the same equipment and procedures. In our experiment, it is assumed that the uncertainty in the measurements is due to the random errors. In such a case, the measure of the uncertainty (random error) in the mean value will be the standard deviation of the mean, called the standard error, which is the precision of the mean, where σx is a standard deviation that can be calculated using the following equation. The standard deviation is a measure of the precision of the measurement. It gives the probability that the experimental data fall within a certain range of the measured mean. Probability theory states that approximately 68.3% of all repeated measurements should fall within a range of plus or minus σx from the mean; 95.5% of all repeated measurements should fall within a range of 2 · σx from the mean. This distribution of the measurements can be displayed graphically in a histogram. A histogram is a bar graph in which the different measured values divided into convenient intervals (or bins), all of the same size, are plotted along the horizontal axis, and the frequency of their occurrence (number of measurements in each interval) is indicated on the vertical axis. As the number of measurements increases and the bin size decreases, the histogram becomes a quite smooth, regular, and continuous curve as shown in Fig. 2.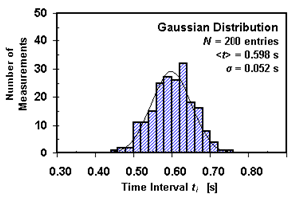
Figure 2
An example of a histogram and a normal distribution for the measurements of time
Video
View the video below prior to beginning your lab.Procedure
Please print the worksheet for this lab. Answer all the questions on the Lab Worksheet prior to submitting the Inlab in WebAssign.Checkpoint:
Be sure to have your TA sign your lab worksheet, printed InLab, and all printed graphs after each part is completed. Be sure the data can be seen on the graphs.
Be sure to have your TA sign your lab worksheet, printed InLab, and all printed graphs after each part is completed. Be sure the data can be seen on the graphs.
Part 1
1
Open the Graphical Analysis (GA) program. The default file consists of a two-column table and a graph area. Double-click on the first column name and change its label to "length" with units "cm." In the same way, change the name and units of the second column to "width" and "cm," respectively.
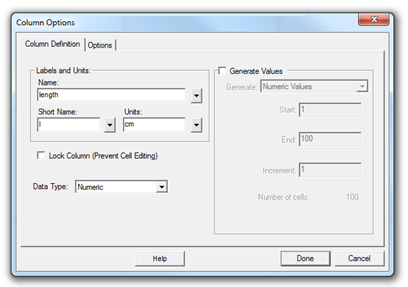
Figure 3
2
Measure the length of the laboratory table with the unmarked wooden rod provided on the table. To make measurements, use only one hand. Do not make any marks on the table with a pen or a finger. You need to have a total of 30 quick, seemingly nonchalant measurements of each dimension. Remember the length of one rod is 20 cm. Make the measurement in centimeters and estimate each measurement of length up to 1 cm.
Report your measurements in cm in GA in the correponding column. (Do not worry about over- or underestimating the measured quantity; the mean value should be the most probable value for your measurements anyway.)
3
To present the distribution of collected data graphically, make a histogram. From the pull-down Insert menu, select Additional Graphs and then Histogram.
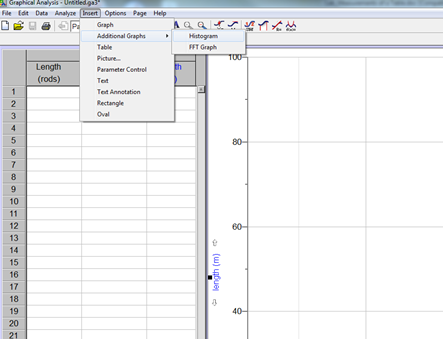
Figure 4
Change histogram features by double-clicking the histogram window. In the open histogram dialog window, you can select the "Bin Size" and the "Bin Start." Play around with these settings to see how they affect the display. Press the STAT button.
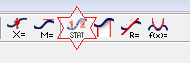
Figure 5
The statistical value including mean values and standard deviation will appear in the box. Record these values in Table 1 in the WebAssign Inlab.
4
Repeat steps 2 and 3 to obtain the mean value of the width of the table as well as the standard deviation. Record these values in Table 1 in the WebAssign Inlab.
5
Using your experimental data, you will need to find the estimated area of the table, along with the uncertainty. To achieve the objectives of this lab, you will also need to find the percentage of data that fits within one and two standard deviations from the mean value of the length to see if the collected data resembles normal distribution. Be sure to report all your results with the correct number of significant figures and in the correct format.
6
On the histogram, count the number of measurements that fall within one standard deviation from the mean and the number of measurements that fall within two standard deviations from the mean. Record both numbers in the WebAssign Inlab. Calculate the percentage of data that fits within one and two standard deviations from the mean value of length and width to see if the collected data resembles normal distribution.
Part 2: Measured Area of a Table
7
Measure the length and width of the table with measuring tape or a 2-meter ruler. (Ask your TA for the measuring instrument.) Record the length and the width of the table with the correct number of significant figures in Table 2 in the WebAssign Inlab. The uncertainy of the measurement equals the smallest division of the instrument.
8
Calculate the measured area of the table and its uncertainty.
9
Complete the group discussion on the Lab Worksheet.
10
Resize and rearrange the three graph windows (length vs. length; width vs. width; length histogram) to fit all on one page. Capture the screen and paste it into a MS Word document. Print it for your TA to sign. Save this file for your future reference and upload it in the WebAssign Inlab. Be sure you can read all the data provided to you on the graphs.
Hint:
First, remember to report only one significant figure in the uncertainty. Secondly, report the mean value and its uncertainty to the same number of decimal places. The rules of handling significant figures can also be found in the document Basic Concepts of Error Analysis or the PowerPoint presentation Results and Uncertainties that is available under Lab Materials on Blackboard for your course.
First, remember to report only one significant figure in the uncertainty. Secondly, report the mean value and its uncertainty to the same number of decimal places. The rules of handling significant figures can also be found in the document Basic Concepts of Error Analysis or the PowerPoint presentation Results and Uncertainties that is available under Lab Materials on Blackboard for your course.
11
Complete the WebAssign Inlab. Be sure you save your entries as you work. Be sure to submit your Inlab by the due date posted on WebAssign.




